Patents
Literature
56results about How to "Little hemolysis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
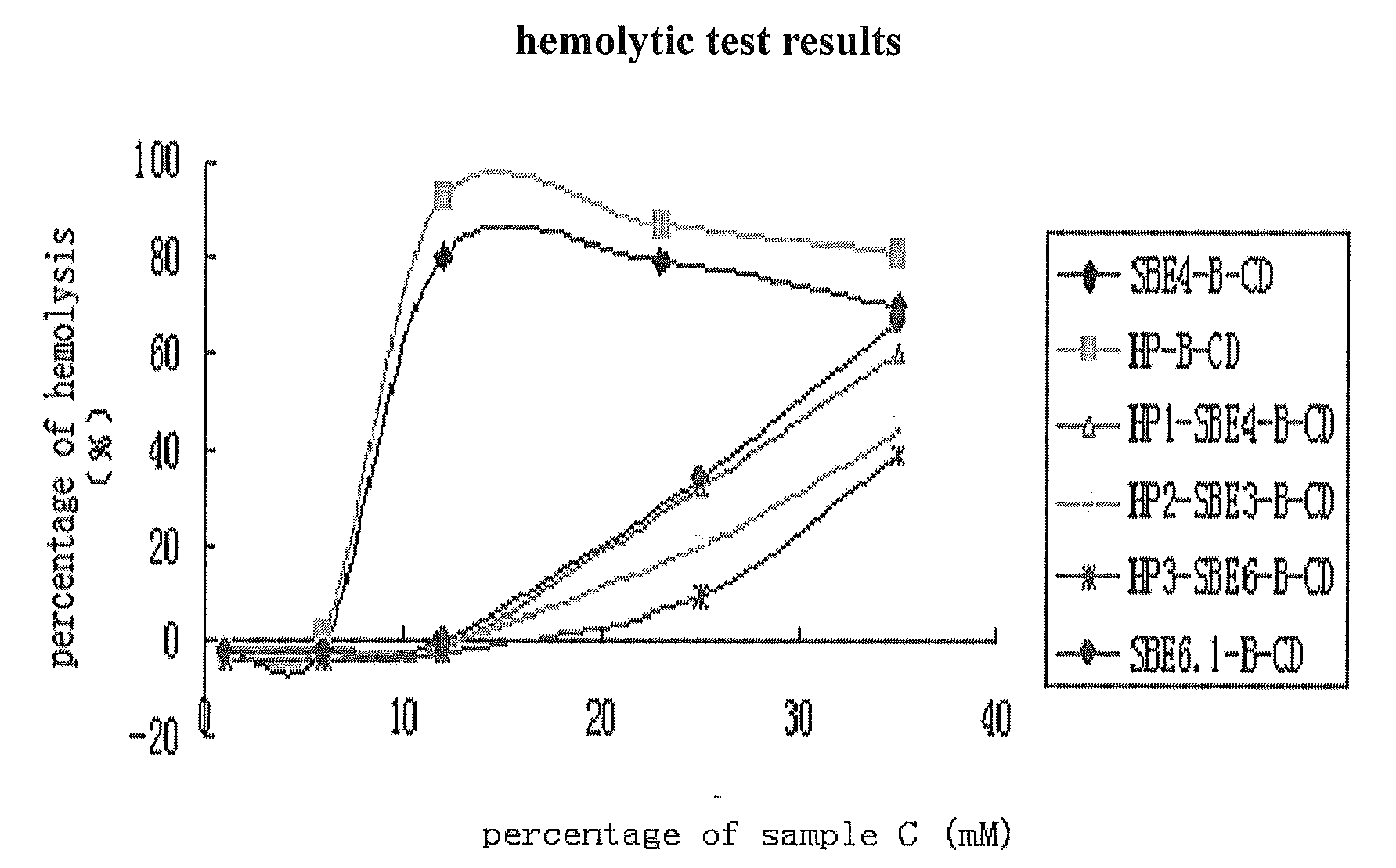
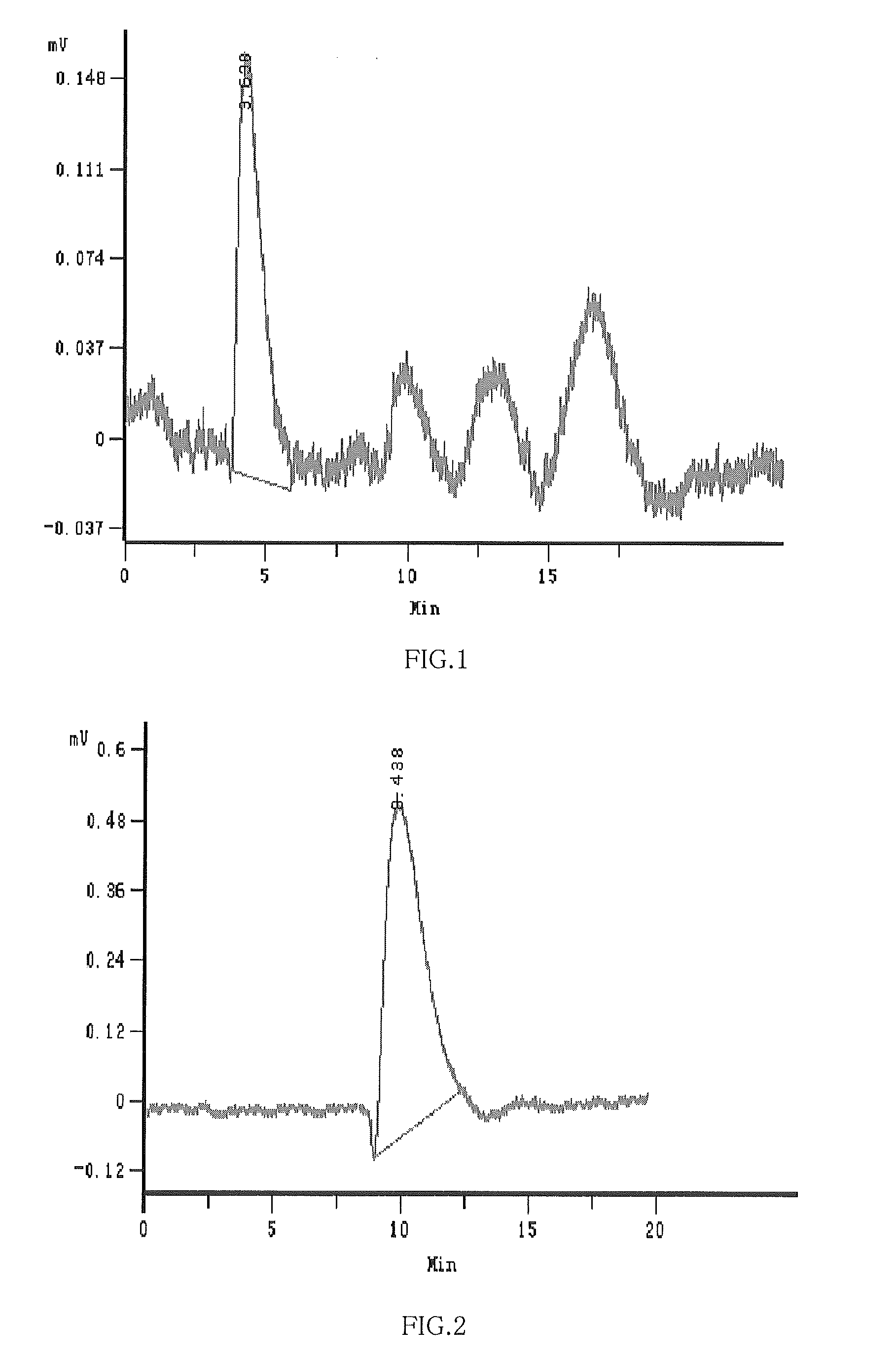
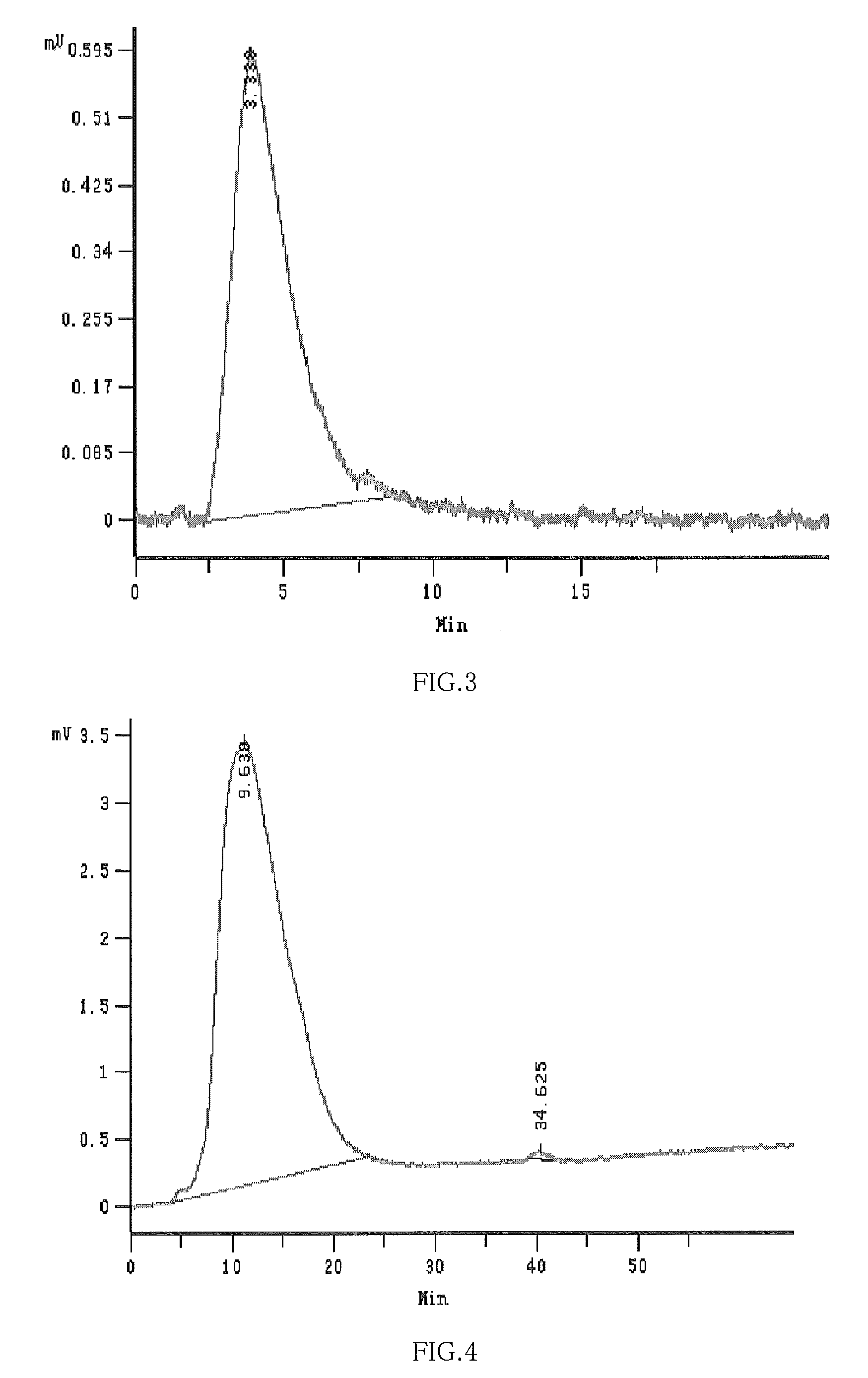
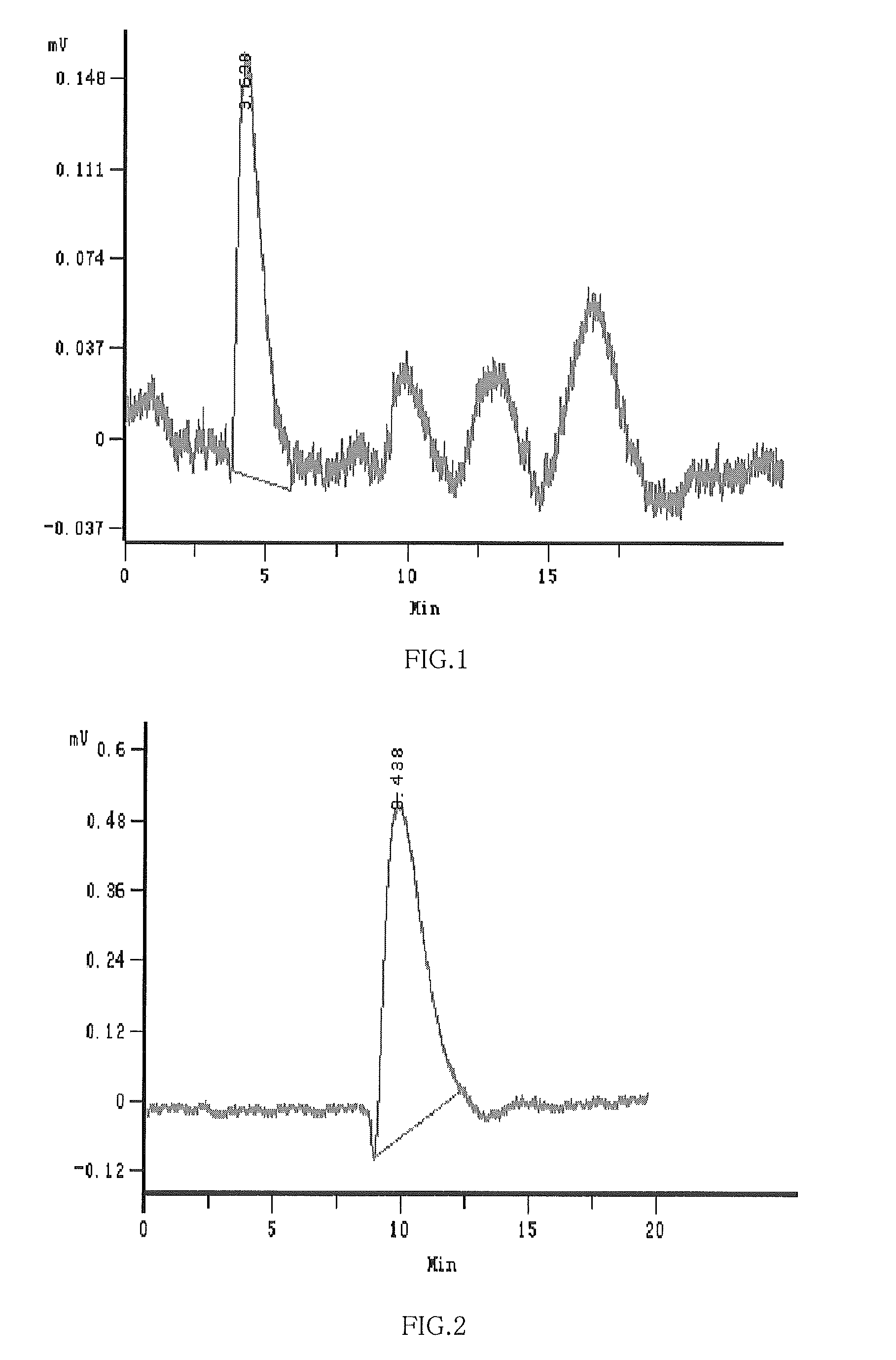
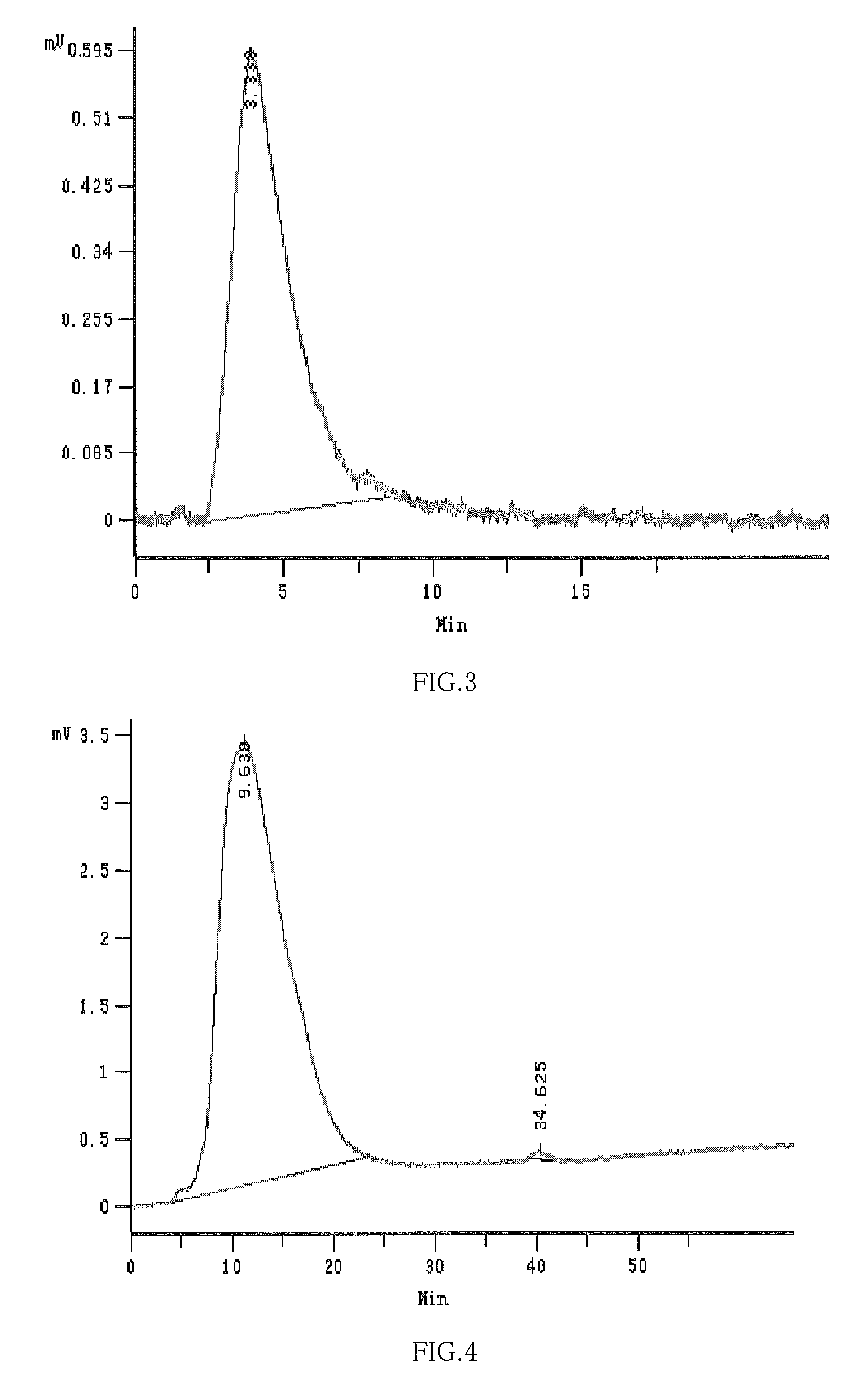
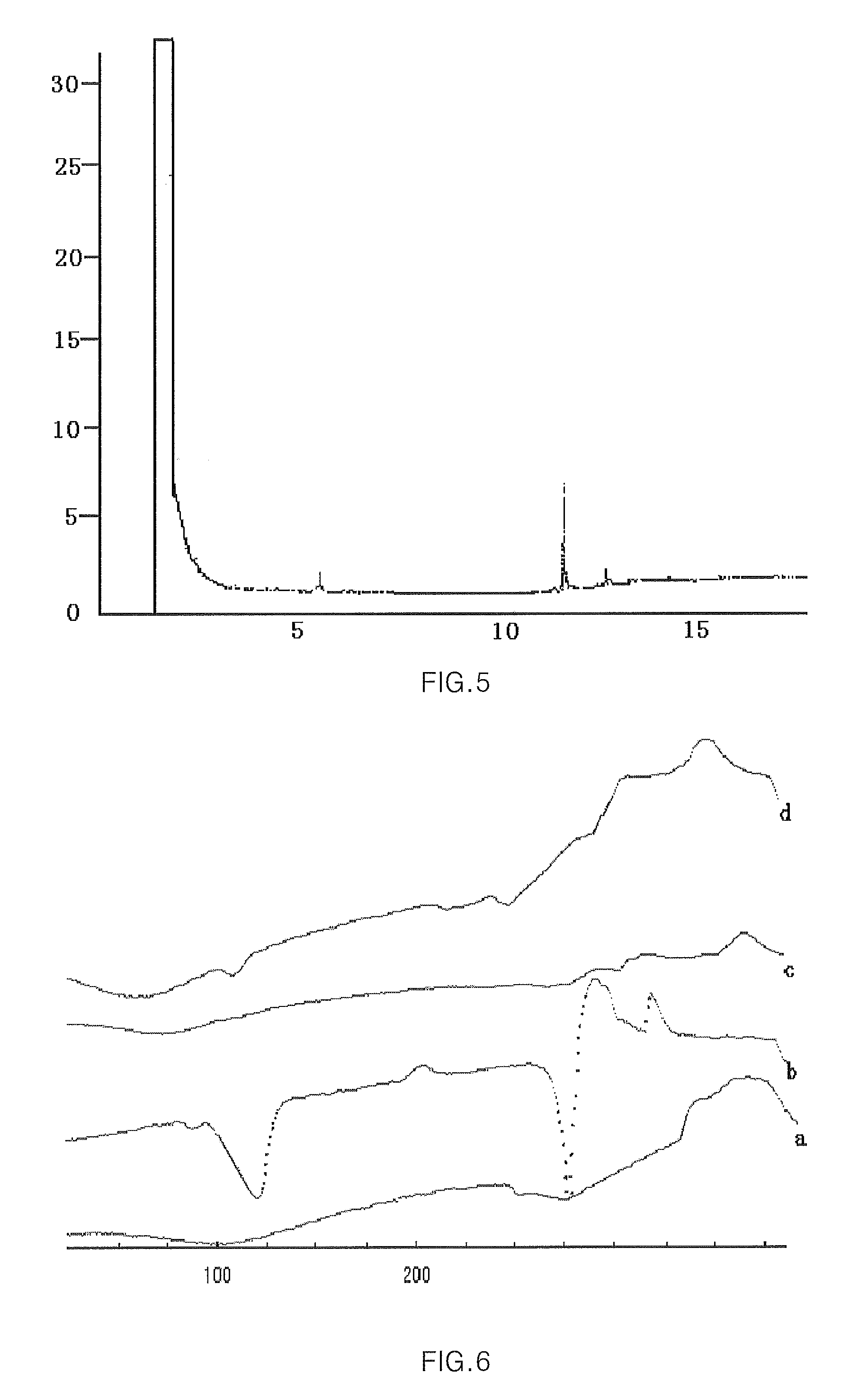
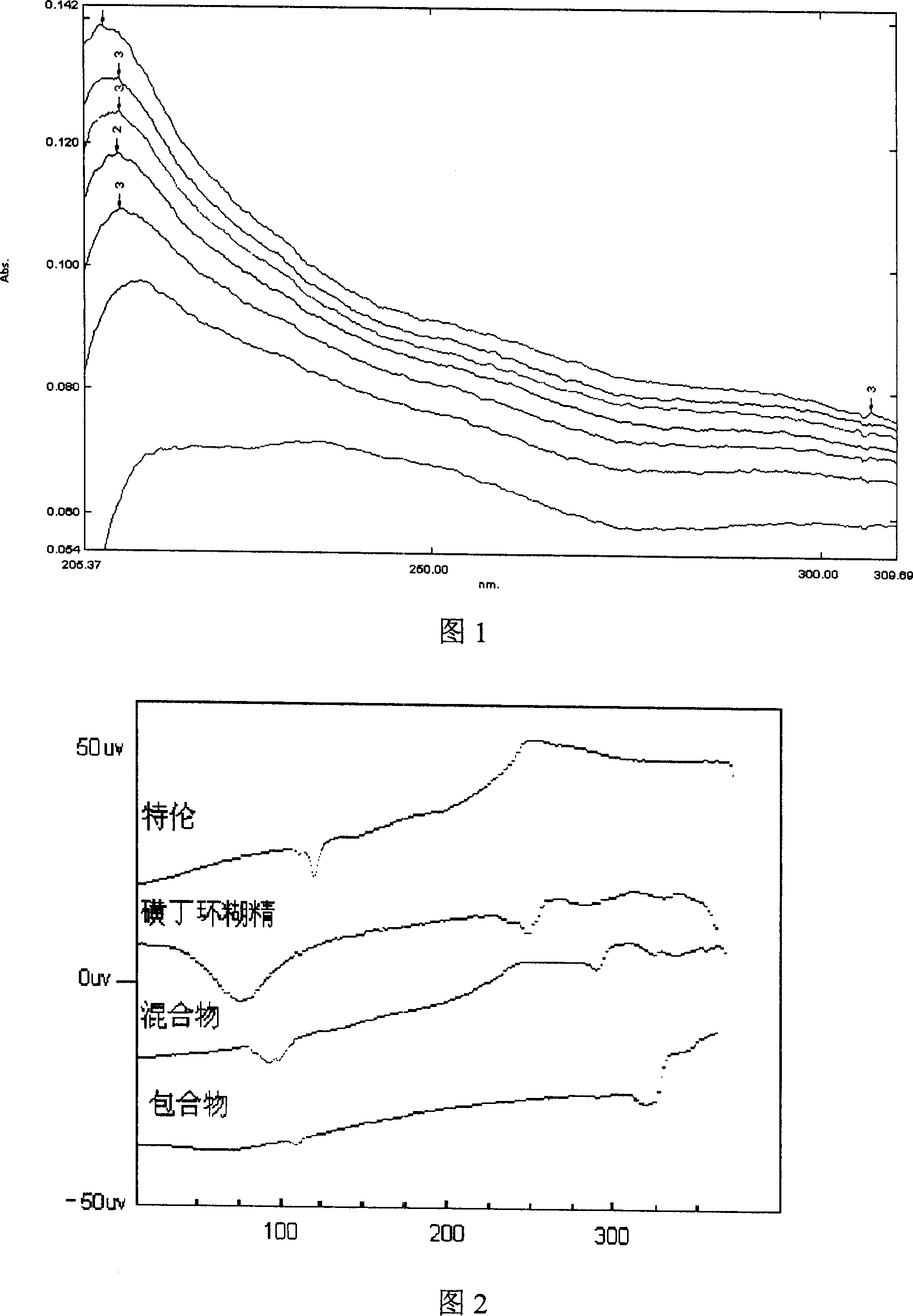
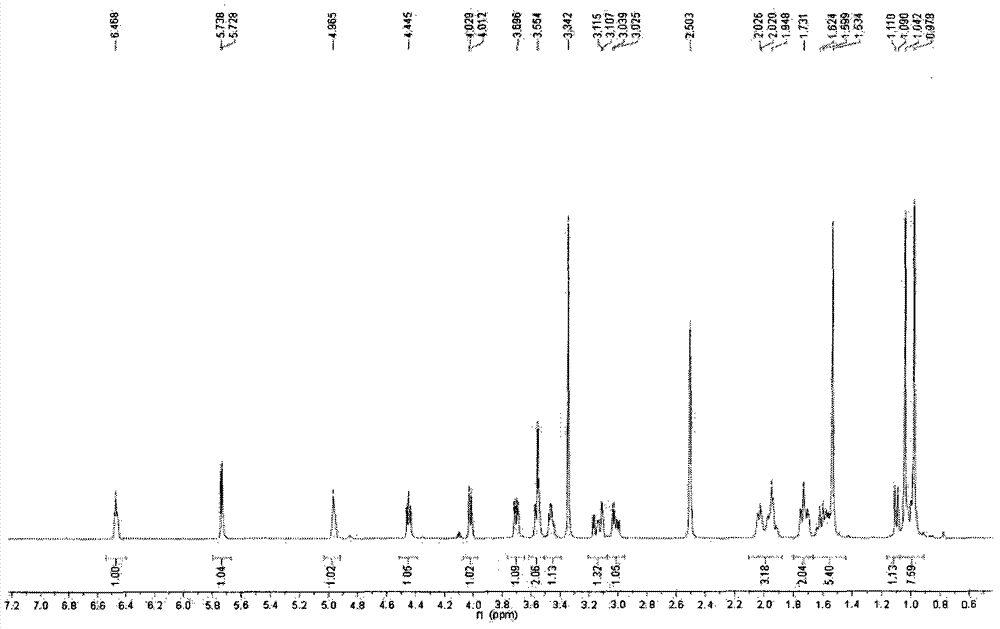
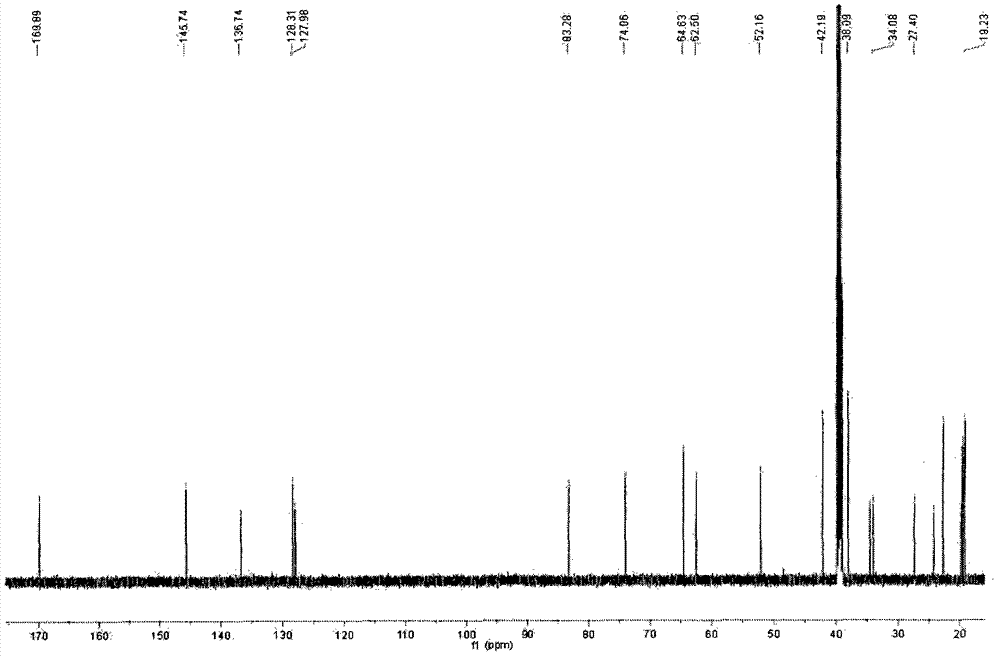
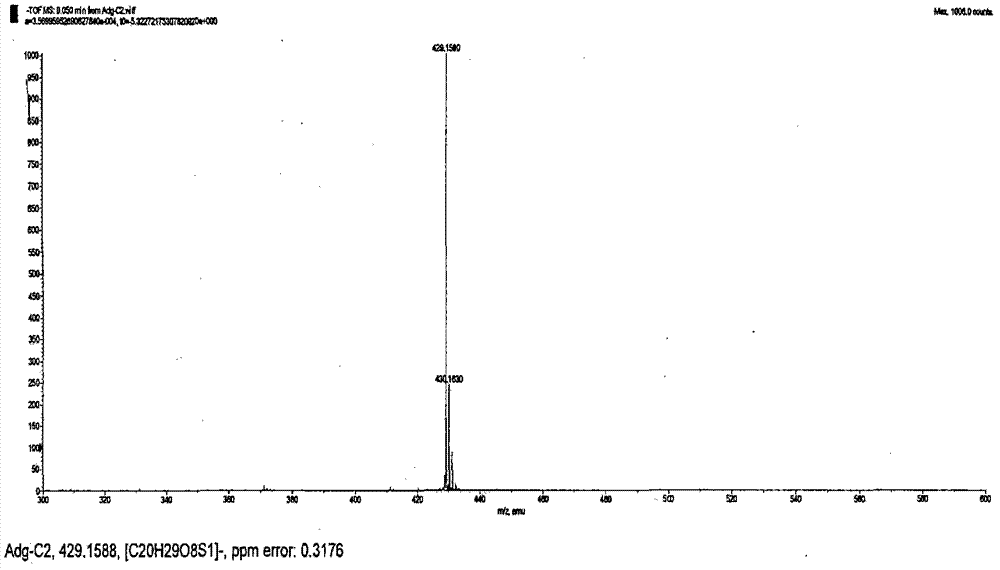
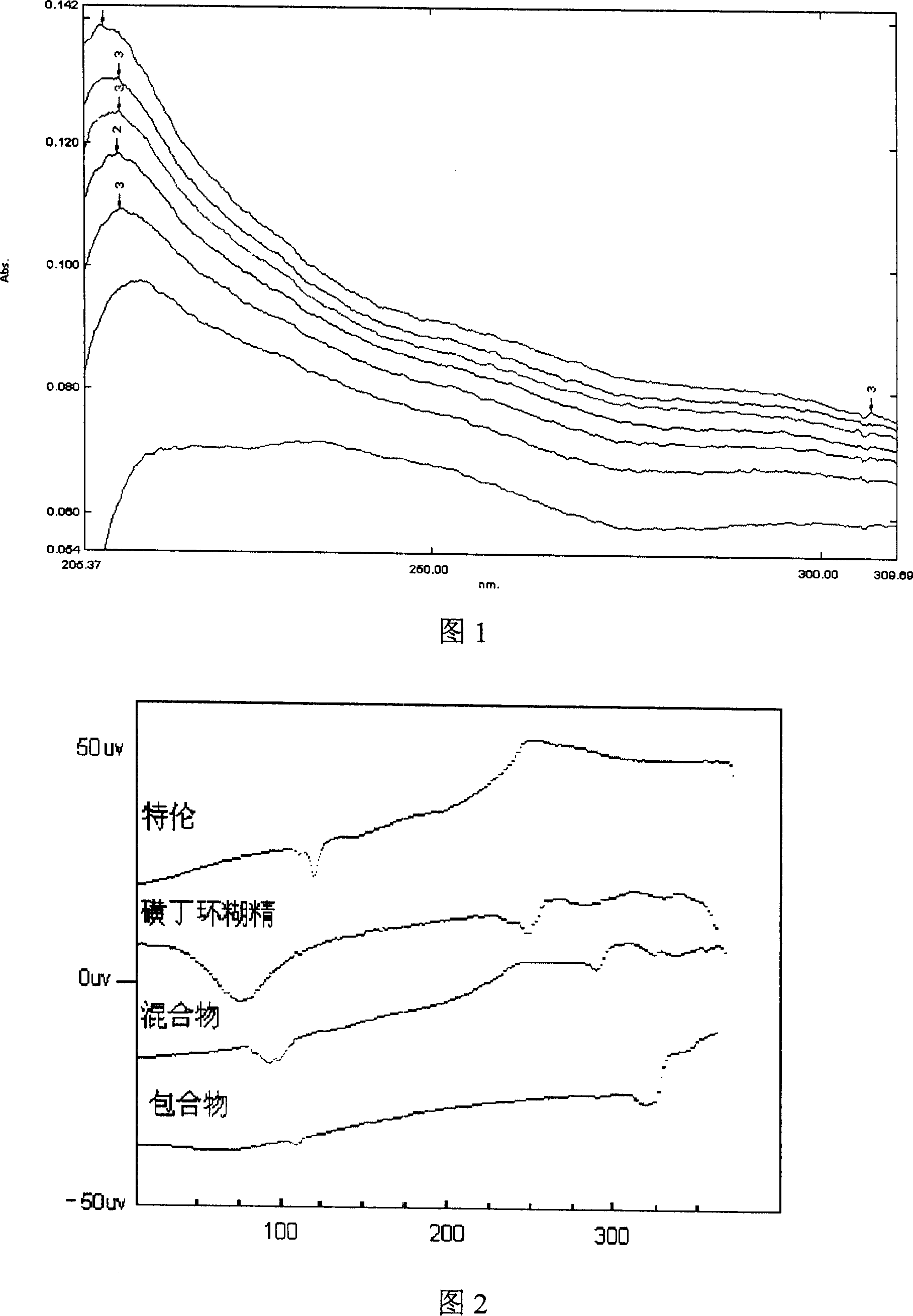
Hydroxypropyl-Sulfobutyl-Beta-Cyclodextrin, the Preparation Method, the Analytical Method, and the Pharmacutical Application Thereof
ActiveUS20090012042A1Little hemolysisLow toxicityBiocideOrganic active ingredientsHaemolysisCyclodextrin
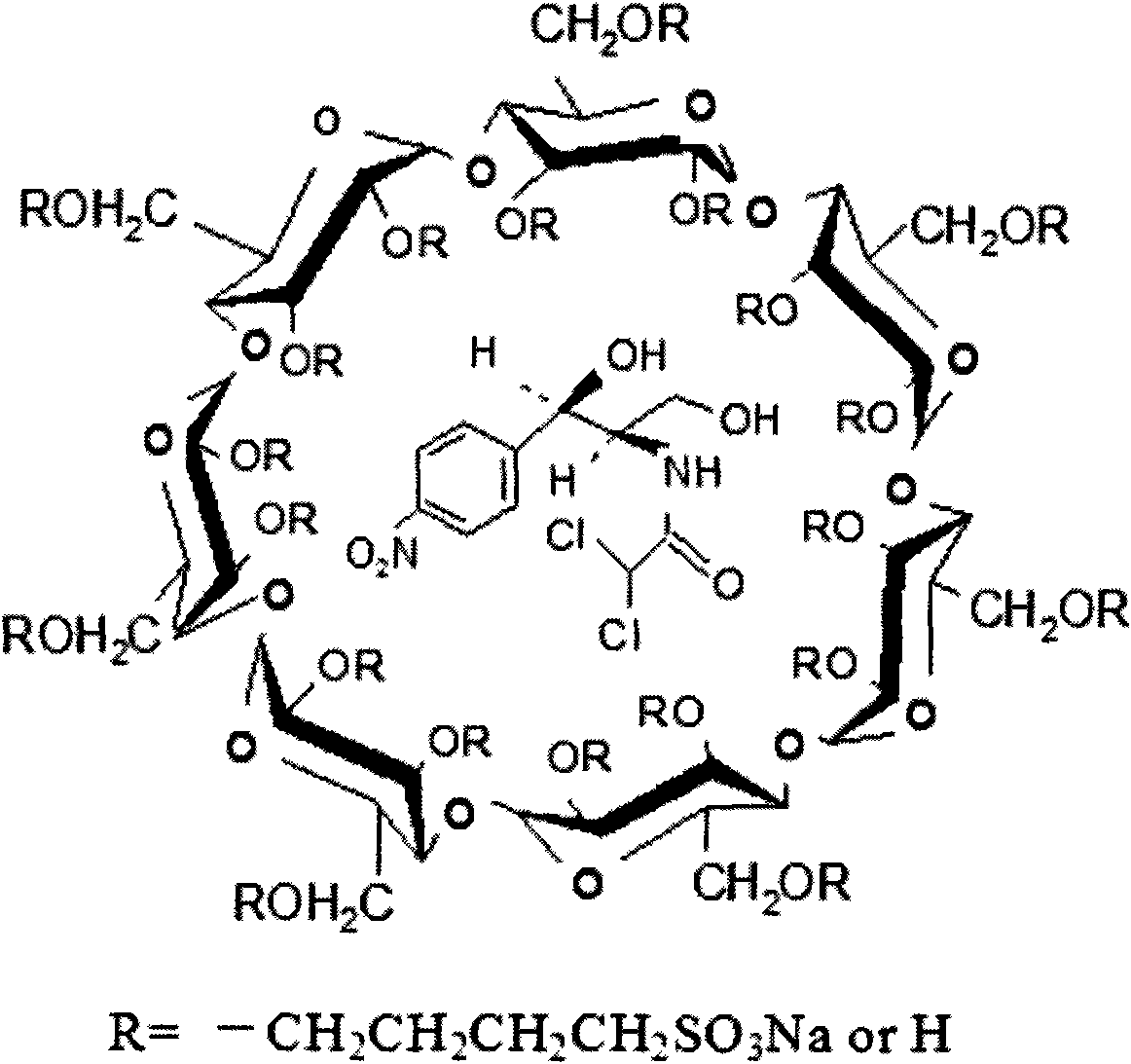
Hydroxypropyl-sulfobutyl-&bgr;-cyclodextrin, the preparation method, analytical method, and the pharmaceutical application thereof. The hydroxypropyl-sulfobutyl-&bgr;-cyclodextrin is a derivate of cyclodextrin which is substituted by hydroxypropyl group and sulfobutyl group: n-(2,3,6-O-2-hydroxypropyl)-m-(2,3,6-O-sulfobutyl)-&bgr;-cyclodextrin. The number of substituent groups per mole cyclodextrin is n hydroxypropyl groups and m sulfobutyl groups. “n” represents the average substituent degree of hydroxypropyl groups; “m” represents the average substituent degree of sulfobutyl groups; “n+m=z” is the gross average substituent degree, in which n is a random integer chosen from 1, 2, 3, 4, 5, 6, 7, 8, 9; m is a random integer chosen from 1, 2, 3, 4, 5, 6, 7, 8, 9; and the gross average substituent degree z is a random integer chosen from 2, 3, 4, 5, 6, 7, 8, 9, 10. The present invention shows low haemolysis and low toxicity.
Owner:SUN XIAODONG
Hydroxypropyl- sulfobutyl-beta- cyclodextrin and its preparation method, analytical method and pharmaceutical uses
ActiveCN1800221ALow hemolyticHemolyticOrganic active ingredientsComponent separationPharmaceutical drugCyclodextrin Derivatives
The invention relates to a hydroxypropyl -sulfo butyl -ª‰-cyclodextrin and its preparing method, analysis method and the applying in medicine, wherein the hydroxypropyl -sulfo butyl -ª‰-cyclodextrin is the cyclodextrin derivation displaced by hydroxypropyl and sulfo butyl. The n-(2, 3, 6-O-2-hydroxypropyl )-m-(2, 3, 6-O-sulfo butyl)-ª‰-cyclodextrin, each mole cyclodextrin displaced group number is n hydroxypropyl and m sulfo butyl, wherein n is the average displaced degree of the hydroxypropyl displaced group; m is the average displaced degree of the sulfo butyl displaced group; n+mú¢Z is the total average displaced degree; nú¢any of the 1,2,3,4,5,6, 7,8,9; m=any of the 1,2,3,4,5,6, 7,8,9; z=any of the 1,2,3,4,5,6, 7,8,9,10.
Owner:BIKA BIOTECH GUANGZHOU CO LTD
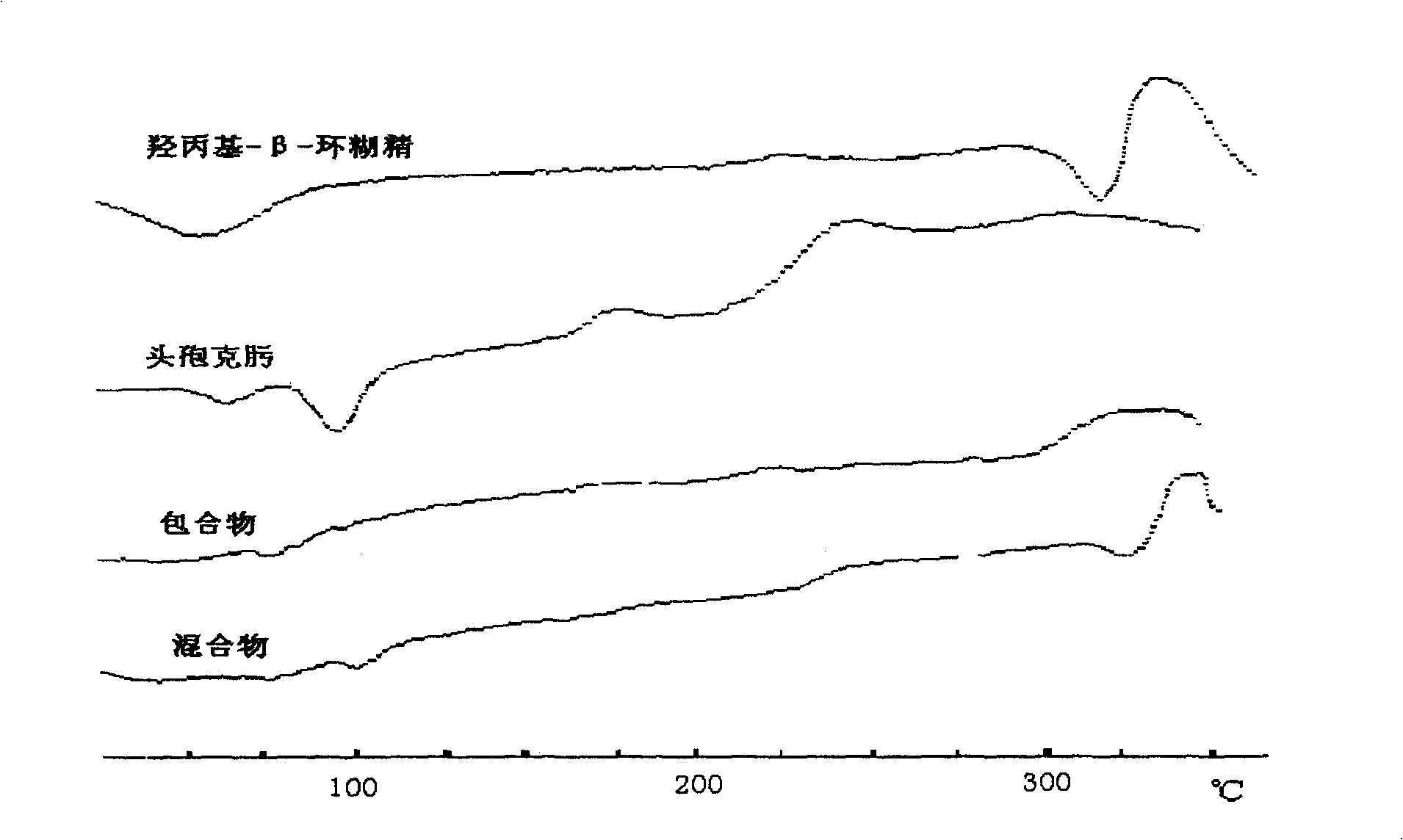
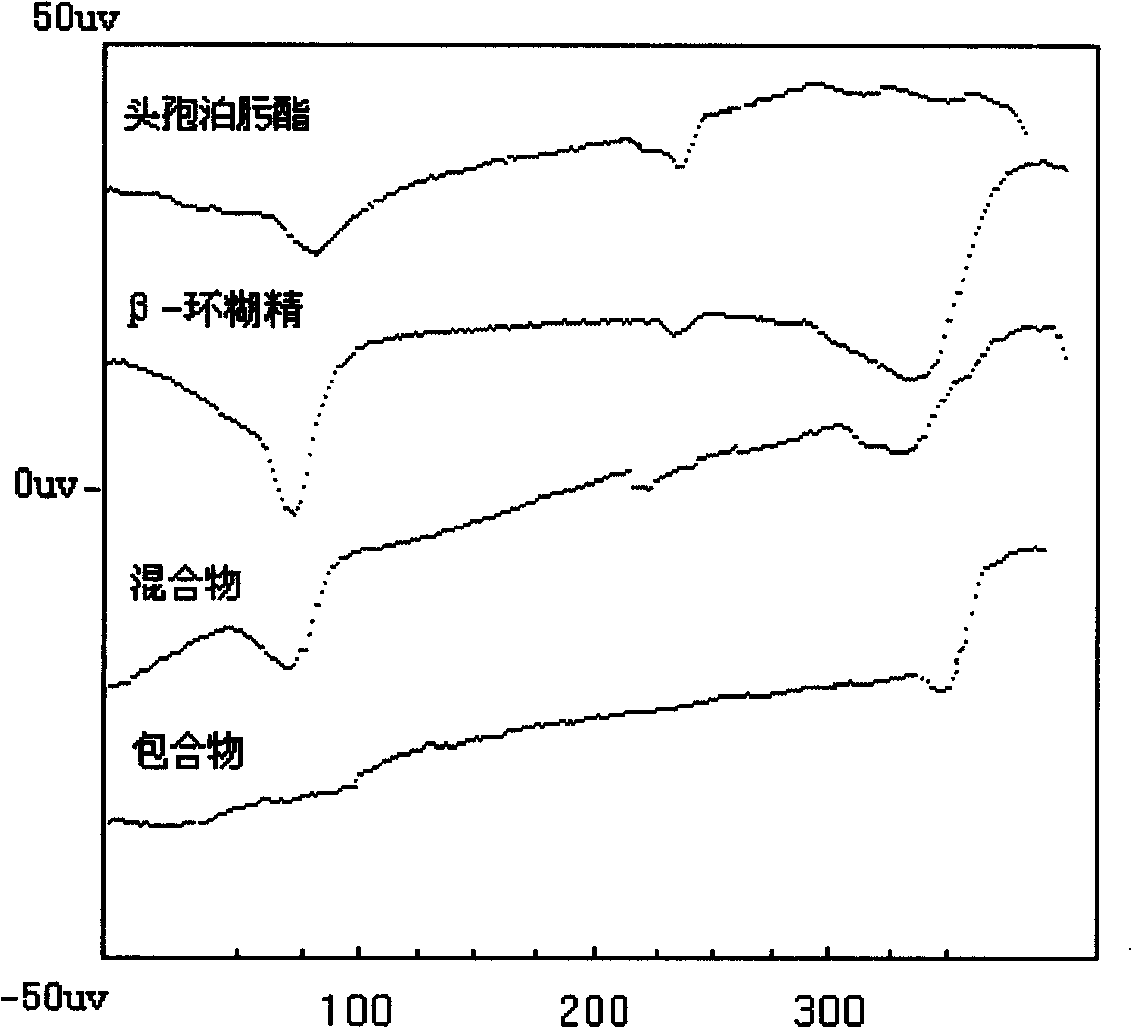
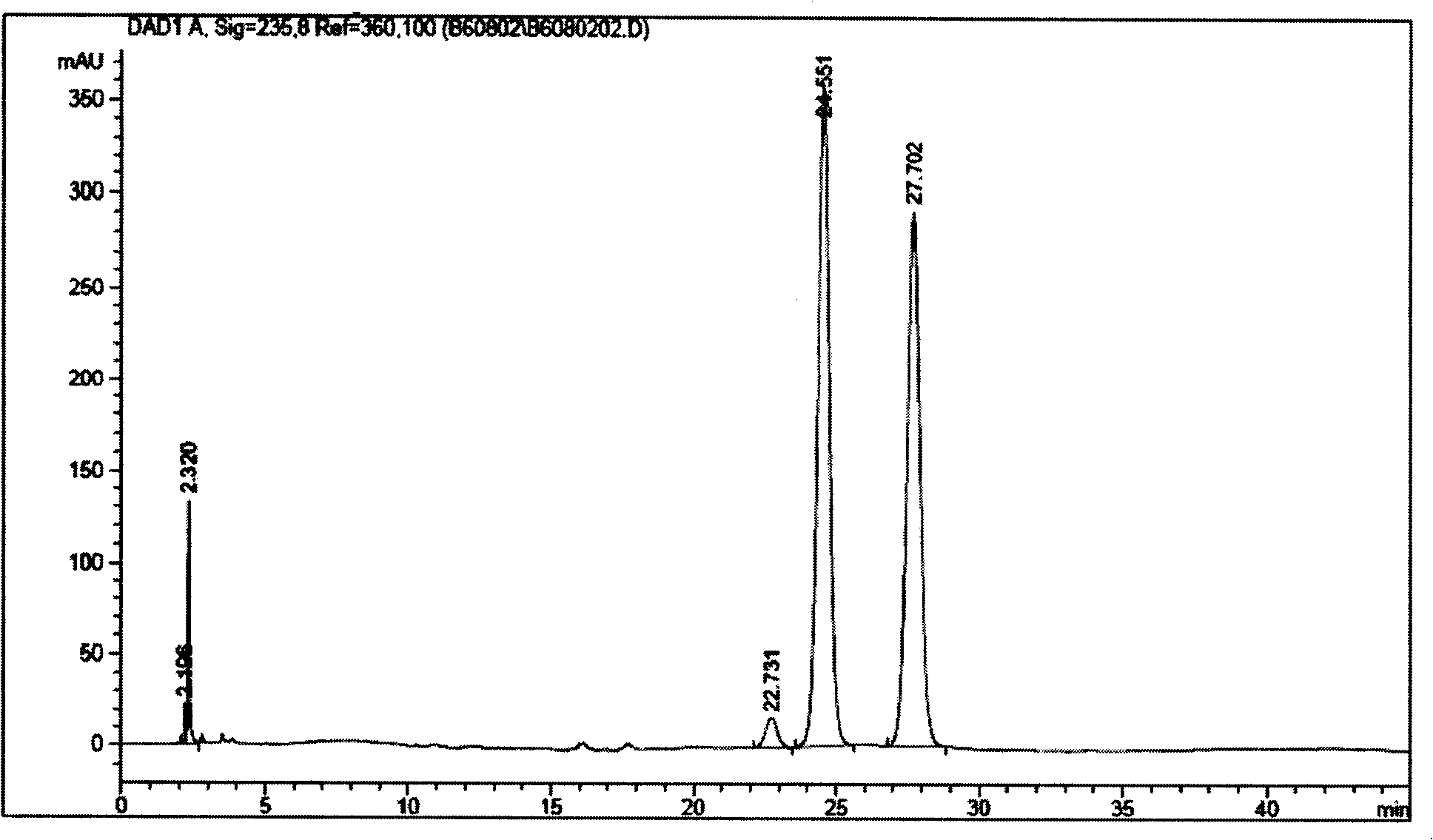
Medicine composition containing ceftin cyclodextrin clathrate, and its preparing method
InactiveCN101002782AImprove water solubilityEasy to dissolveAntibacterial agentsOrganic active ingredientsSolubilityAlpha-Cyclodextrin
An inclusion compound of cefuroxime axetil with high solubility, stability and activity contains cefuroxime axetil and the pharmacologically acceptable cyclodextrin chosen from alpha-cyclodextrin, beta-cyclodextrin, gamma-cyclodextrin and their derivatives. Its preparing process is also disclosed.
Owner:海南和德通医药科技信息咨询有限公司
Medicinal composition containing cefdinir cyclodextrin inclusion compound and preparation thereof
InactiveCN101264085AGood water solubilityImprove solubilityAntibacterial agentsOrganic active ingredientsSolubilityCefdinir
The invention relates to a medicine composition comprising the cefdinir cyclodextrin inclusion compound. The basic composition comprises the cefdinir and the pharmacy-acceptable cyclodextrin; the cyclodextrin is chosen from one or a plurality of Beta-cyclodextrin, sulfobutyl- Beta -cyclodextrin, hydroxypropyl- Beta-cyclodextrin or hydroxypropyl-sulfobutyl- Beta -cyclodextrin. The invention has an advantage of increasing the solubility, stability and activity of the cefdinir. The invention also provides the preparation method of the medicine composition.
Owner:NANJING NORMAL UNIVERSITY +1
Hydroxypropyl-sulfobutyl-beta-cyclodextrin, the preparation method, the analytical method, and the pharmacutical application thereof
ActiveUS8278437B2Little hemolysisLow toxicityOrganic active ingredientsBiocideHaemolysisCyclodextrin
Hydroxypropyl-sulfobutyl-&bgr;-cyclodextrin, the preparation method, analytical method, and the pharmaceutical application thereof. The hydroxypropyl-sulfobutyl-&bgr;-cyclodextrin is a derivate of cyclodextrin which is substituted by hydroxypropyl group and sulfobutyl group: n-(2,3,6-O-2-hydroxypropyl)-m-(2,3,6-O-sulfobutyl)-&bgr;-cyclodextrin. The number of substituent groups per mole cyclodextrin is n hydroxypropyl groups and m sulfobutyl groups. “n” represents the average substituent degree of hydroxypropyl groups; “m” represents the average substituent degree of sulfobutyl groups; “n+m=z” is the gross average substituent degree, in which n is a random integer chosen from 1,2,3,4,5,6,7,8,9; m is a random integer chosen from 1,2,3,4,5,6,7,8,9; and the gross average substituent degree z is a random integer chosen from 2,3,4,5,6,7,8,9,10. The present invention shows low haemolysis and low toxicity.
Owner:SUN XIAODONG
Medicine composition containing cefateram cyclodextrin capsule and its preparing method
InactiveCN100998595AImprove water solubilityEasy to dissolveAntibacterial agentsOrganic active ingredientsDrugIrritation
A composite medicine with high solubility, stability and activity and low hemolytic irritation contains cefteram ester and the pharmacologically acceptable dextrin chosen from beta-cyclodextrin and its derivatives. Its preparing process is also disclosed.
Owner:海南和德通医药科技信息咨询有限公司
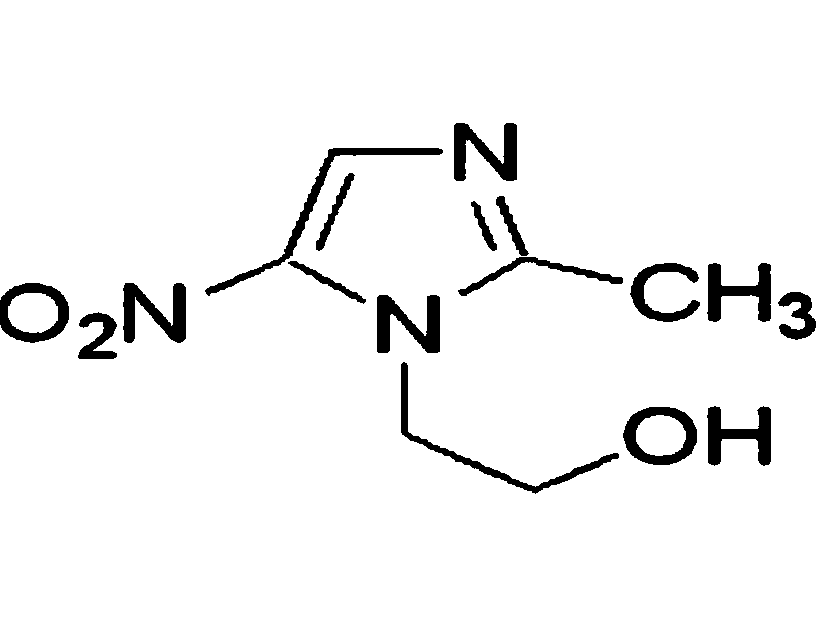
Nitrofurazone washing fluid, preparation method and application thereof
InactiveCN102210651AEasy to useThe scope of clinical application is smallAntibacterial agentsOrganic active ingredientsSolventPatient compliance
The invention discloses a novel nitrofurazone washing fluid, which mainly comprises an active component nitrofurazone, a solubilizing agent polyethyleneglycol 15-hydroxyl stearate and a solvent injection water. Furthermore, one or more of cosolvent, pH regulator, osmotic pressure regulator and stabilizing agent can be added into the washing fluid. In the washing fluid, a novel solubilizer Solutol HS 15 has low haemolysis, extremely low histamine liberation and higher physiological tolerance, so that safety of clinical administration and compliance of patients are remarkably improved; the washing fluid has excellent stability and longer validity, and higher administration convenience is given to clinical doctors; and the washing fluid has better storage and transportation stability, higher clinical administration safety and patient compliance. The washing fluid has a simple preparation process, convenience in quality control, lower production cost and industrial production.
Owner:85 HOSPITAL OF PEOPLES LIBERATION ARMY
Fosfomycin calcium composition freeze-dried orally disintegrating tablets and preparation method thereof
InactiveCN102784118ADisintegrates quicklyPromote dissolutionAntibacterial agentsOrganic active ingredientsFreeze-dryingOrally disintegrating tablet
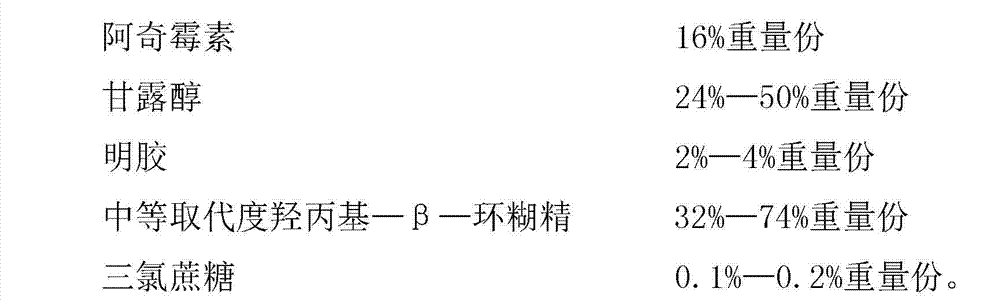
The invention discloses fosfomycin calcium composition freeze-dried orally disintegrating tablets and a preparation method of the orally disintegrating tablets, relates to the field of a medicine and a medicine preparation method technology and mainly solves the problems in the prior art that the oral preparation made from fosfomycin calcium is poor in administration compliance for children and calcium salt orally taking absorption rate. The fosfomycin calcium composition freeze-drying orally disintegrating tablets are prepared from the following components by weight: 10-24% of fosfomycin calcium, 24-50% of mannitol, 2-4% of gelatin, 32-74% of hydroxypropyl-beta-cyclodextrin with medium substitution degree, 0.1-0.2% of trichlorosucrose and 50% of purified water. The fosfomycin calcium composition freeze-drying orally disintegrating tablets obtained by the above components are easy in preparation process, and simple in components, and mainly aim at the administration requirements of the children; moreover, the fosfomycin calcium composition freeze-drying orally disintegrating tablets have the advantages of being fast to disintegrate, convenient to take and good in taste; and the tablets take effect quickly and are fully absorbed, and the first pass effect of the liver is avoided.
Owner:HAINAN WEI KANG PHARMA QIANSHAN
Stable butylphthalide sodium chloride injection as well as preparation method and application thereof
PendingCN112386571AImprove solubilityGood water solubilityOrganic active ingredientsInorganic non-active ingredientsSodium Chloride InjectionCyclodextrin
The invention discloses a stable butylphthalide sodium chloride injection as well as a preparation method and application thereof, belongs to the technical field of pharmaceutical preparations, and solves the problems of poor safety and stability of the butylphthalide sodium chloride injection in the prior art. The stable butylphthalide sodium chloride injection comprises a sulfobutyl betacyclodextrin sodium compound, sodium chloride and water. The pH value of the butylphthalide sodium chloride injection is 4.0-5.0. The preparation method comprises the following steps of weighing a prescription amount of sulfobutyl betacyclodextrin sodium, and adding water for dissolving to prepare an auxiliary material solution; weighing a prescription amount of butylphthalide, adding the butylphthalide into the auxiliary material solution, stirring to enable the sulfobutyl betacyclodextrin sodium to include the butylphthalide, and adjusting the pH value of the solution to 4.0-5.0 after the inclusionof the butylphthalide is completed; and filtering, filling and sterilizing to obtain the product. The stable butylphthalide sodium chloride injection as well as the preparation method and applicationthereof are scientific in design and ingenious in thought, and the butylphthalide sodium chloride injection has good stability and safety.
Owner:CHENGDU SHIBEIKANG BIOLOGICAL MEDICINE TECH CO LTD
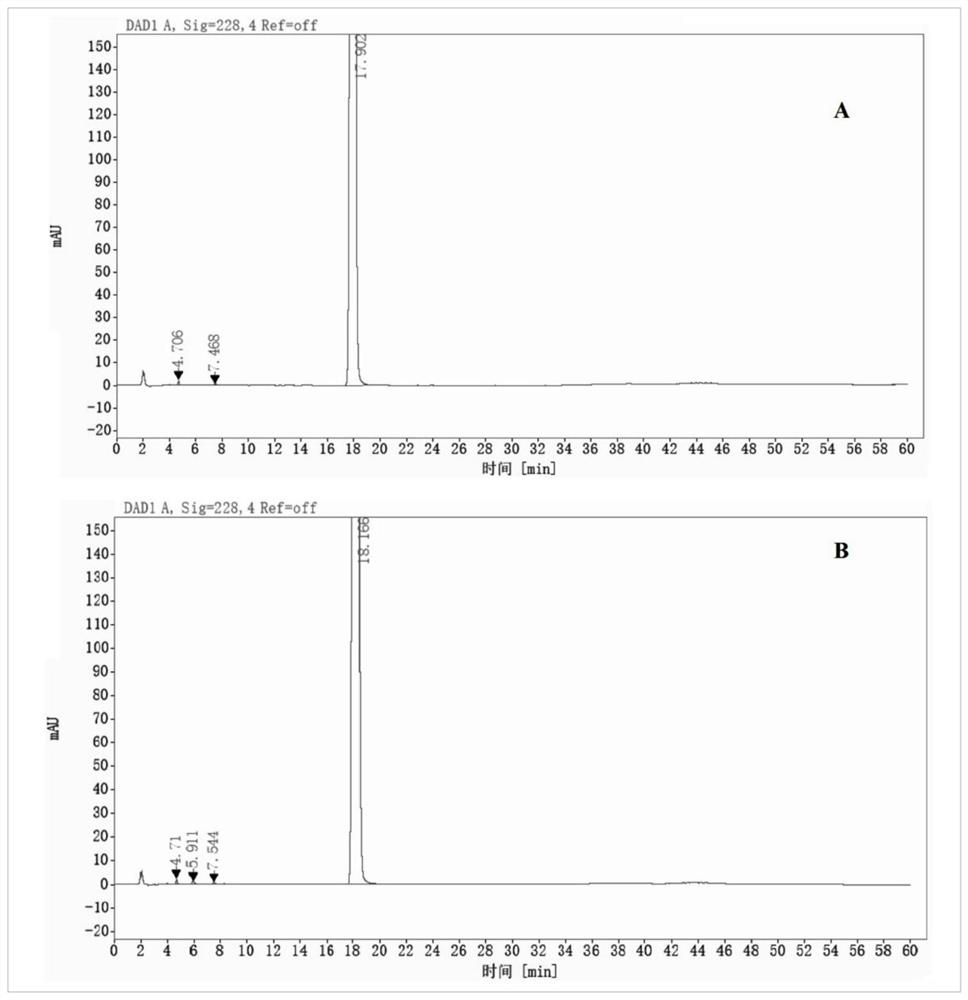
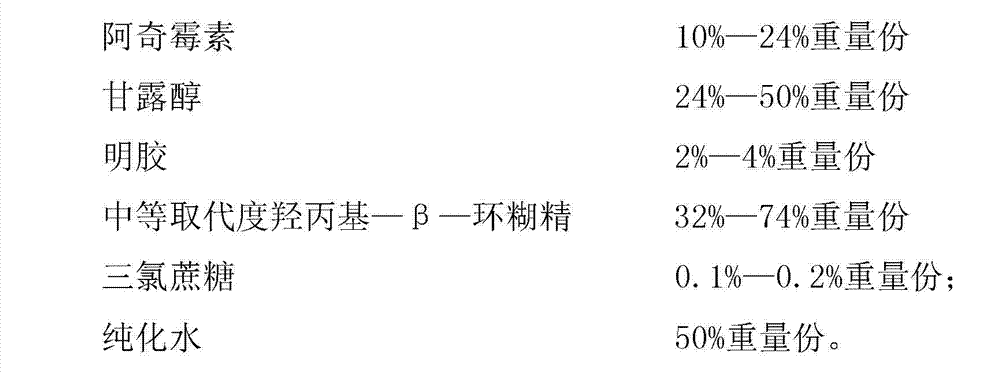
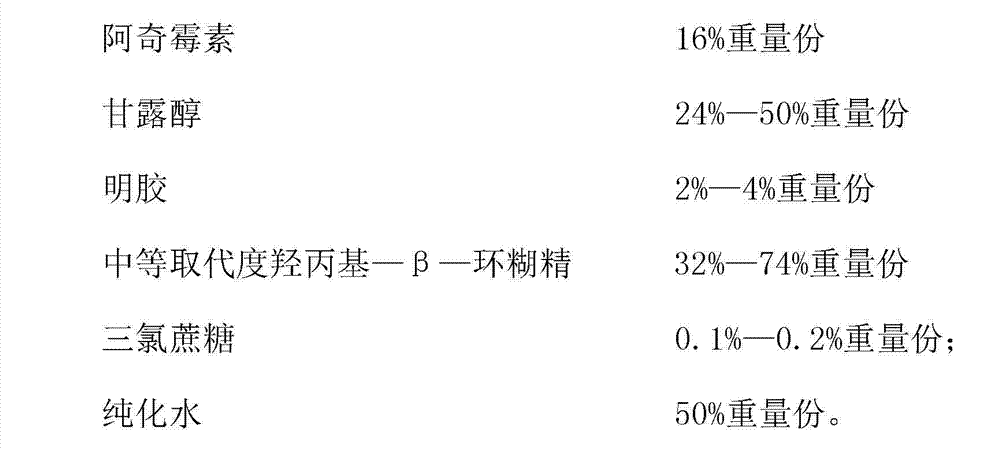
Azithromycin composition freeze-dried orally disintegrating tablet and preparation method thereof
InactiveCN102772383ADisintegrates quicklyPromote dissolutionAntibacterial agentsOrganic active ingredientsAzithromycinSide effect
The invention discloses an azithromycin composition freeze-dried orally disintegrating tablet and a preparation method thereof, relates to the technical field of medicines and preparation methods thereof, and mainly solves the problems that the production process and the azithromycin preparation components are complicated, and the disintegrating tablet is low in disintegrating speed in the prior art. The azithromycin composition freeze-dried orally disintegrating tablet comprises the following components in percentage by weight: 10 to 24 percent of azithromycin, 24 to 50 percent of mannitol, 2 to 4 percent of gelatin, 32 to 74 percent of middle-substitution-degree hydroxypropyl-beta-cyclodextrin, 0.1 to 0.2 percent of sucralose and 50 percent of purified water. The preparation process of the azithromycin composition freeze-dried orally disintegrating tablet prepared by the components is simple, and the components are simple; and the azithromycin preparation can reduce the side effect of azithromycin, is convenient to take and tastes good.
Owner:HAINAN WEI KANG PHARMA QIANSHAN
Metronidazole composition freeze-dried disintegrating tablets for vaginas and preparation method thereof
ActiveCN102784120ADisintegrates quicklyPromote dissolutionAntibacterial agentsOrganic active ingredientsEffervescent tabletFreeze-drying
The invention provides metronidazole composition freeze-dried disintegrating tablets for vaginas and a preparation method of the freeze-dried disintegrating tablets, relates to the field of a medicine and a medicine preparation method technology and mainly solves the technical problems that the metronidazole suppositories and effervescent tablets are inconvenient to transport and store, the application is limited; and the inflamed mucosas are frequently stimulated by the metronidazole suppositories and effervescent tablets when metronidazole suppositories and effervescent tablets are utilized, and the clinical application is influenced. The metronidazole composition freeze-dried disintegrating tablets are prepared from the following components by weight: 10-24% of metronidazole, 24-50% of mannitol, 2-4% of gelatin, 32-74% of hydroxypropyl-beta-cyclodextrin with medium substitution degree and 50% of purified water. The metronidazole composition freeze-dried disintegrating tablets for vaginas prepared by the above components are easy in preparation process, and simple in components, moreover, the metronidazole composition freeze-dried disintegrating tablets for vaginas have the advantages of being fast to disintegrate, convenient to take, and good in compliance, and the tablets take effect quickly and are fully absorbed; and the disintegrating tablets can be completely disintegrated in the vaginas while meeting water, so that foreign body sensation does not exist in the vaginas.
Owner:HAINAN WEI KANG PHARMA QIANSHAN
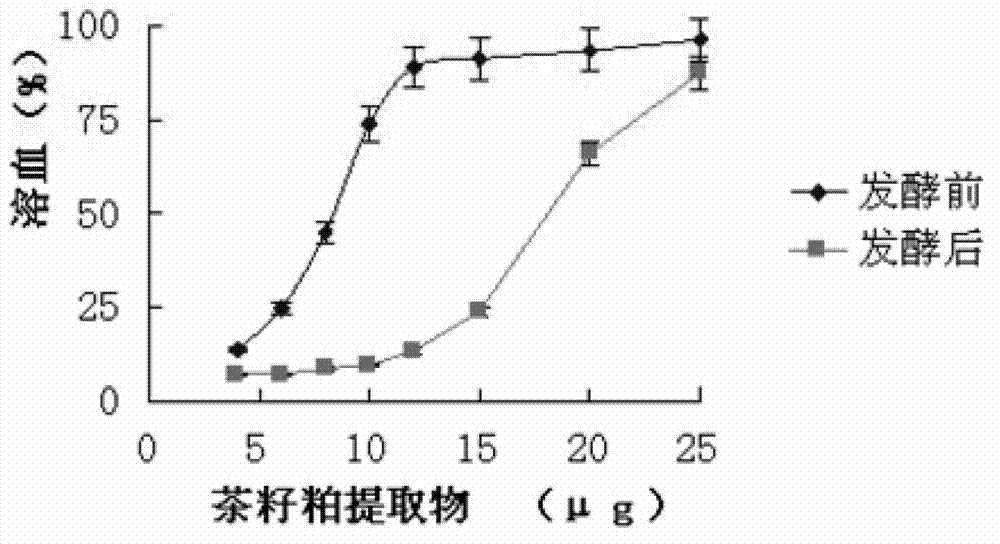
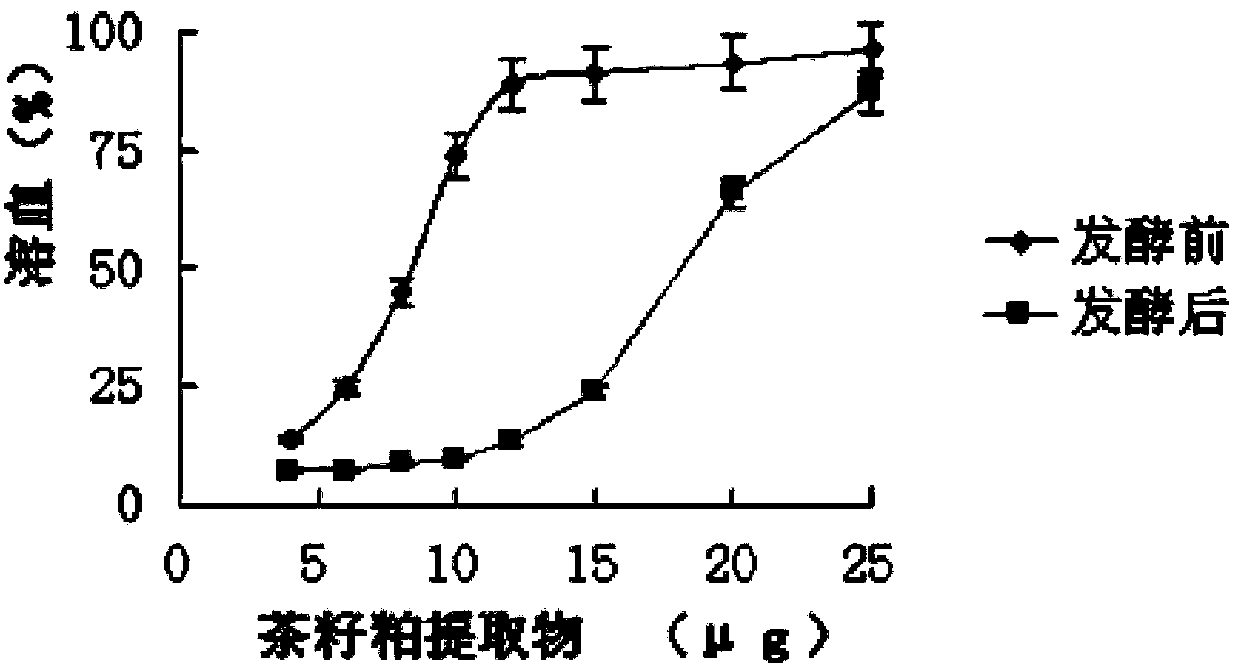
Method for producing camellia oil cake feed through mixed strain fermentation
InactiveCN103039710APromote growth and reproductionLittle hemolysisAnimal feeding stuffChemistryGenus Lactobacillus
The invention relates to a method for producing camellia oil cake feed through mixed strain fermentation. Bread lactobacillus and bacillus natto are inoculated into camellia oil cake and are subjected to aerobic fermentation and anaerobic fermentation to produce low-toxicity feed. The method provided by the invention overcomes the shortcomings of camellia oil cake in feed application, degrades tea saponin and reduces the hemolysis thereof, degrades cellulose in camellia oil cake, produces flavor substance through lactobacillus fementation and improves the palatability of the feed; and moreover, since bacillus natto is a subspecies of bacillus subtilis, the bacillus natto after heating and drying still exists in the fermented camellia oil cake in a form of spores and thus can realize the function of probiotics.
Owner:SHANGHAI JIAO TONG UNIV +1
Levofloxacin composition freeze-dried orally disintegrating tablets and preparation method thereof
InactiveCN102784121ADisintegrates quicklyPromote dissolutionAntibacterial agentsOrganic active ingredientsFreeze dryHepatic first pass effect
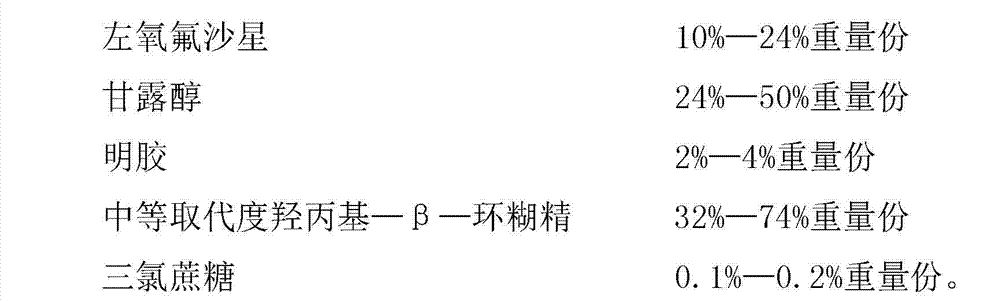
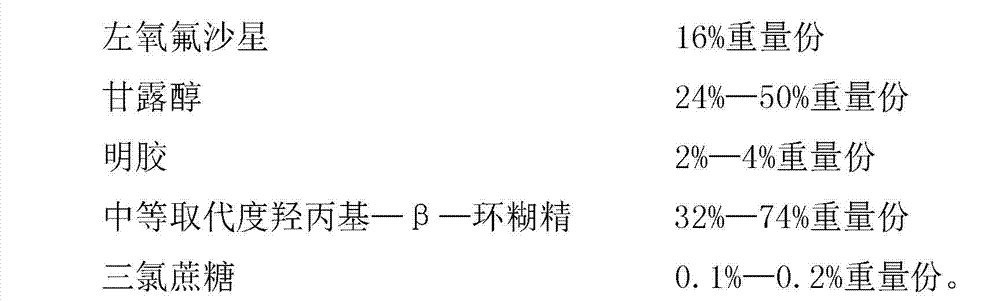
The invention provides levofloxacin composition freeze-dried orally disintegrating tablets and a preparation method of the orally disintegrating tablets, relates to the field of a medicine and a medicine preparation method technology and mainly solves the problems in the prior art that the ordinary preparation prepared by levofloxacin is poor in curative effect and not suitable for children and the patients who are difficult to swallow. The levofloxacin composition freeze-dried orally disintegrating tablets are prepared from the following components by weight: 10-24% of levofloxacin, 24-50% of mannitol, 2-4% of gelatin, 32-74% of hydroxypropyl-beta-cyclodextrin with medium substitution degree, 0.1-0.2% of trichlorosucrose and 50-70% of purified water. The levofloxacin composition freeze-dried orally disintegrating tablets prepared by the above components are easy in preparation process, and simple in components, and mainly meet the administration requirements of children and the patients who are difficult to swallow; moreover, the levofloxacin composition freeze-dried orally disintegrating tablets have the advantages of being fast to disintegrate, convenient to take, and good in taste; and the tablets take effect quickly and are fully absorbed, and the first pass effect of the liver is avoided.
Owner:HAINAN WEI KANG PHARMA QIANSHAN
Composition injection of glycerol and fructose and preparation method thereof
ActiveCN101810629AReasonable prescriptionImprove medication safetySenses disorderHydroxy compound active ingredientsFructoseAdditive ingredient
The invention relates to a composition injection of glycerol and fructose and a preparation method thereof. The composition injection of the glycerol and the fructose comprises the following main ingredients: 25-40 parts of glycerol, 3-8 parts of fructose, 3-13 parts of maltitol and 80-120 parts of water for injection. The composition injection of glycerol and fructose is prepared according to the following steps: (1) liquid preparation: dissolving the main materials and auxiliary materials into proper amount of water for injection to prepare solution, respectively stirring evenly, mixing the prepared solution evenly by stirring, and using a pH regulator to regulate pH to be 4 to 5, wherein hydrochloric acid or sodium hydroxide is preferred as the pH regulator, and filtering to obtain filtrate; (2) sterilization: carrying out high-temperature sterilization on the filtrate in the step (1), and taking proper amount of active carbon for high-temperature sterilization; and (3) decolorization: adding the medical active carbon after high-temperature sterilization into the filtrate after high-temperature sterilization, decolorizing and filtering.
Owner:罗诚
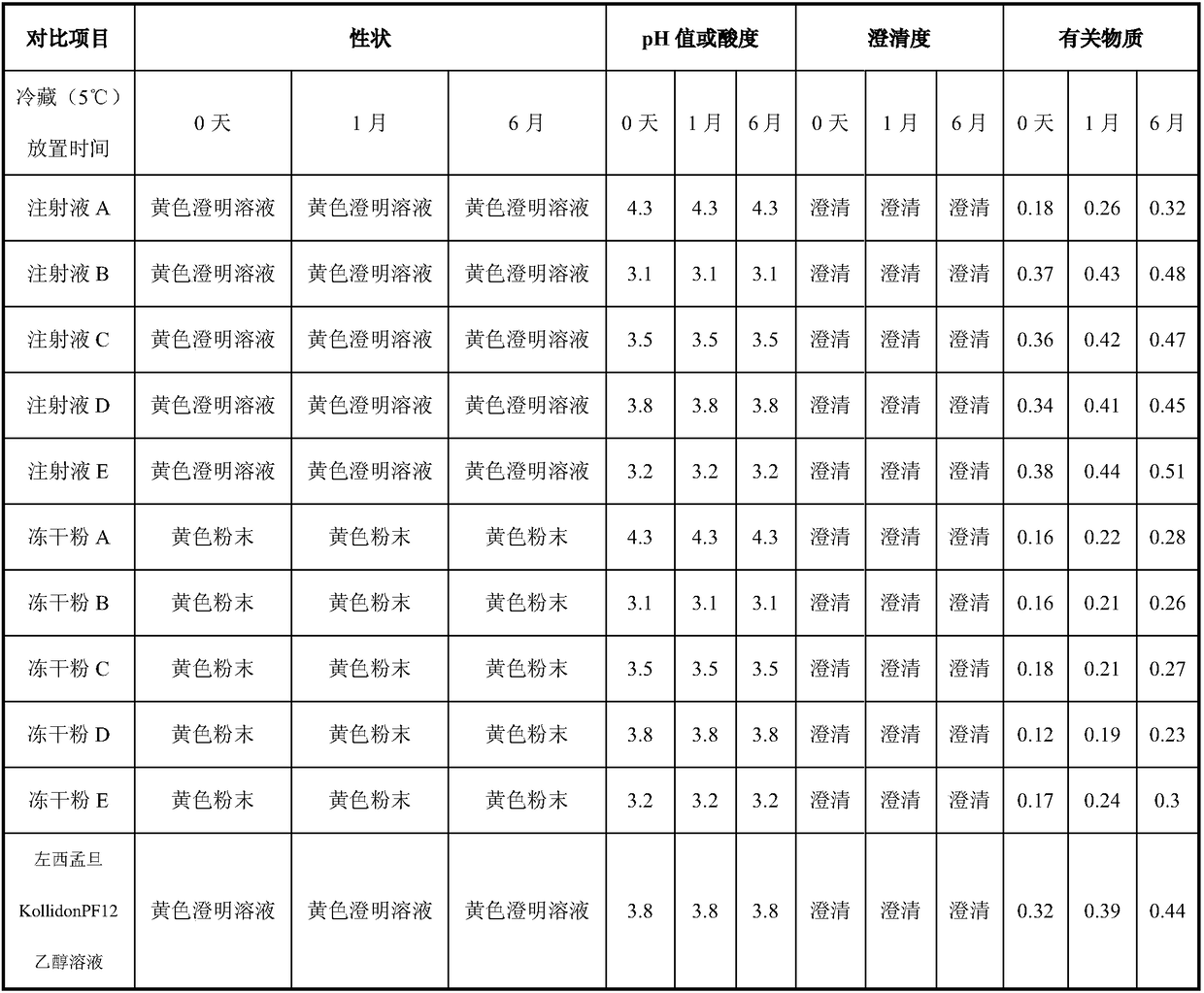
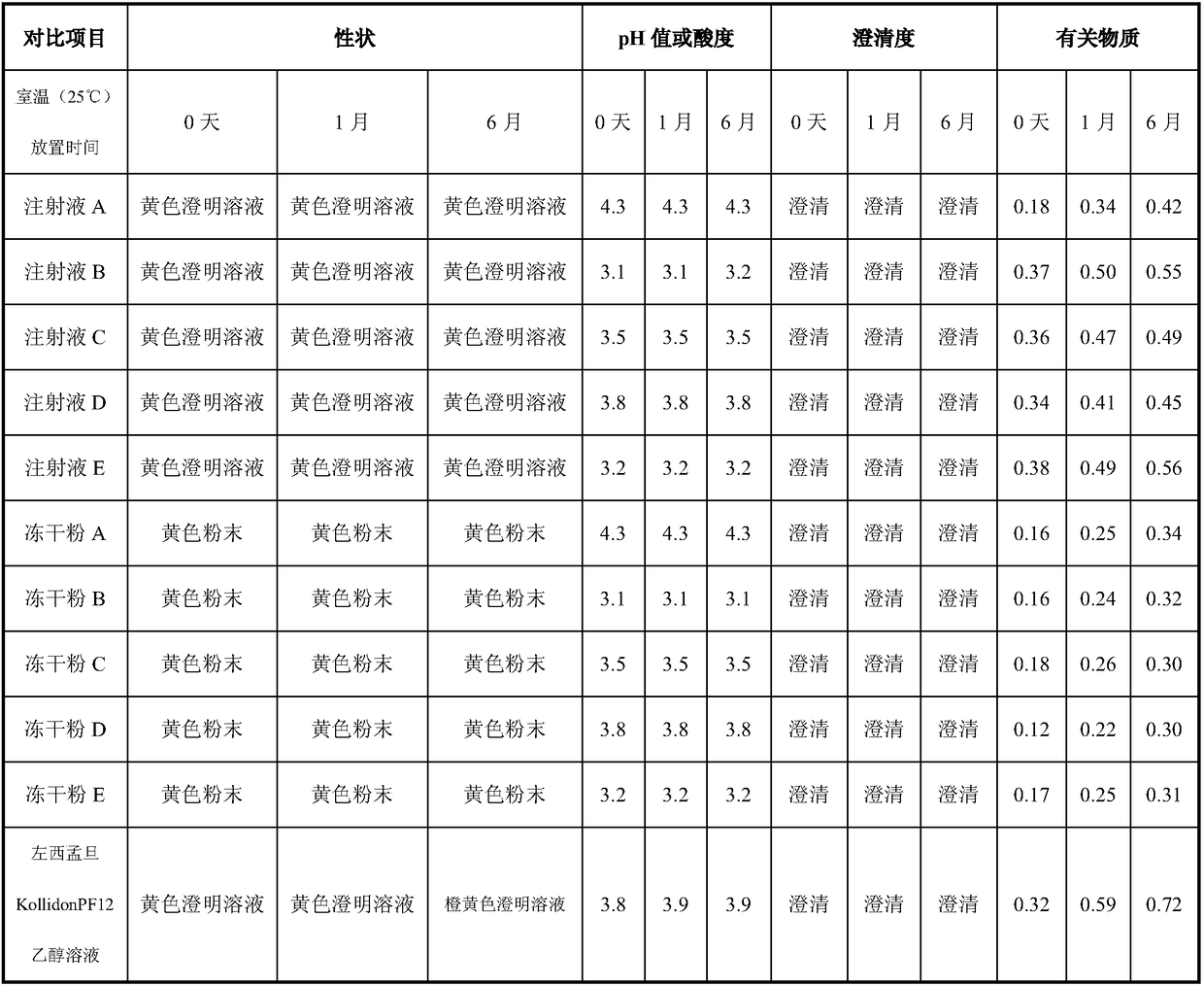
Levosimendan-containing injection medicine preparation and preparation method thereof
InactiveCN108261398AAvoid inconvenienceImprove securityPowder deliveryLyophilised deliveryFreeze-dryingBULK ACTIVE INGREDIENT
The invention provides a levosimendan-containing injection medicine preparation and a preparation method thereof, and belongs to the field of medicine preparations. The preparation can be injection solution or freeze-dried powder and is mainly used for administrating medicines by injection. The injection solution comprises levosimendan serving as an active component or medicinal derivative of thelevosimendan and sulfobutyl beta cyclodextrin serving as a solubilizing stabilizer or medicinal salt of the sulfobutyl beta cyclodextrin, the weight ratio of the active component to the solubilizing stabilizer is 1:10-300, preferably, the weight ratio is 1:40-100, and most preferably, the weight ratio is 1:64-100. According to the preparation, the accessory sulfobutyl beta cyclodextrin has betterwater solubility, less hemolytic action and low renal toxicity as compared with hydroxypropyl beta cyclodextrin and is firstly used as a clathration material of the levosimendan or the medicinal derivative of the levosimendan. Compared with marketed similar injection solution, the injection solution takes water as a solvent, does not contain ethyl alcohol and is better in safety, and a freeze-dried preparation is more stable and easily stored at the room temperature.
Owner:QILU PHARMA CO LTD
Medicinal composition containing cefixime cyclodextrin inclusion compound and preparation thereof
InactiveCN101264086AGood water solubilityImprove solubilityAntibacterial agentsOrganic active ingredientsSolubilityHigh activity
The invention provides a medicine composition comprising the cefixime cyclodextrin inclusion compound. The basic composition comprises the cefixime and the pharmacy-acceptable cyclodextrin; the cyclodextrin is chosen from one or a plurality of Beta -cyclodextrin, sulfobutyl- Beta -cyclodextrin, hydroxypropyl- Beta -cyclodextrin or hydroxypropyl-sulfobutyl- Beta -cyclodextrin. The invention has the advantages of increasing the solubility and stability of the medicine, and higher activity. The invention also provides the preparation method of the medicine composition.
Owner:NANJING NORMAL UNIVERSITY +1
Macromolecule skin penetration enhancer as well as preparation method and applications thereof
ActiveCN103100088AGood water solubilityImprove stabilityPharmaceutical delivery mechanismPharmaceutical non-active ingredientsGlycidyl methacrylateSkin penetration
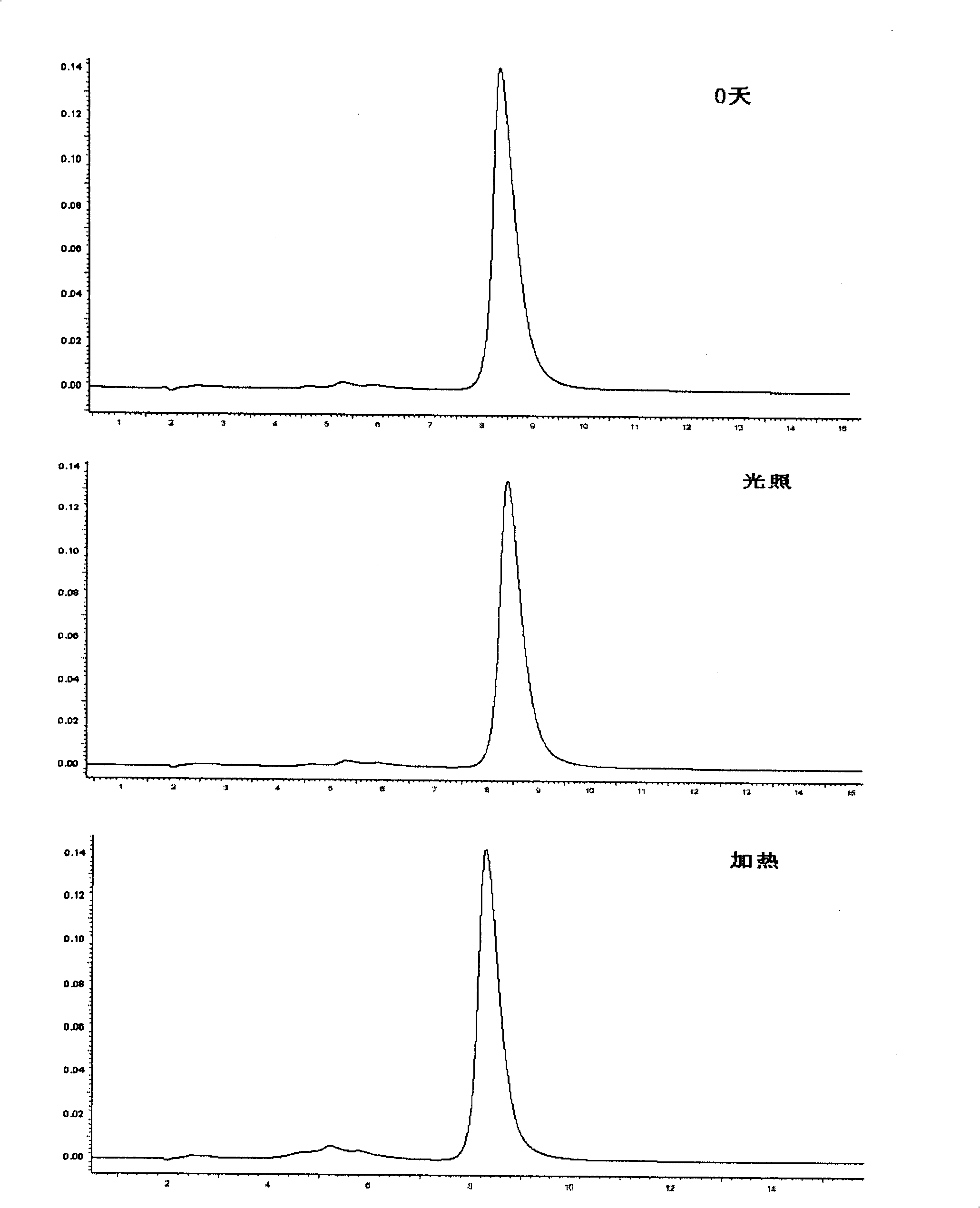
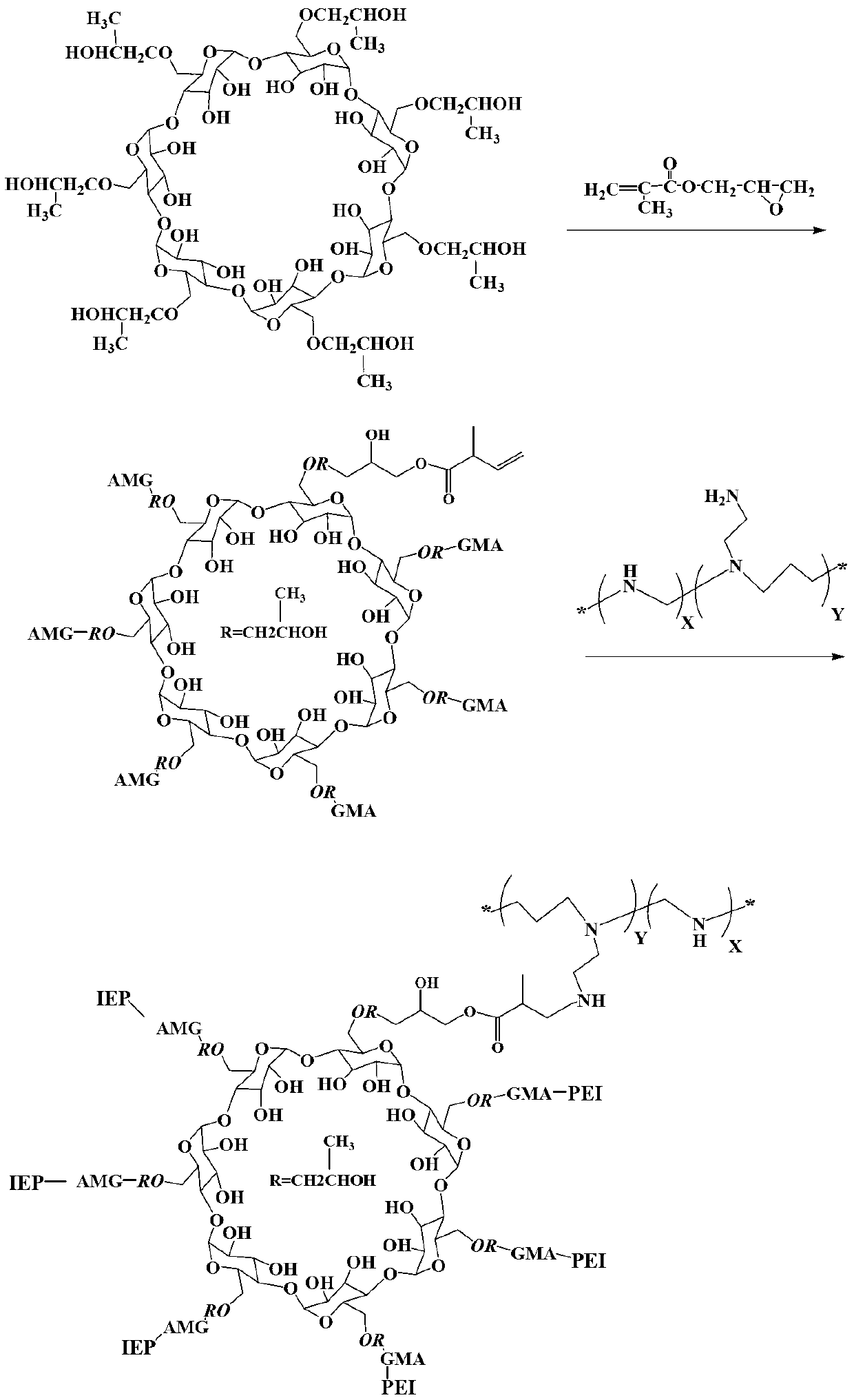
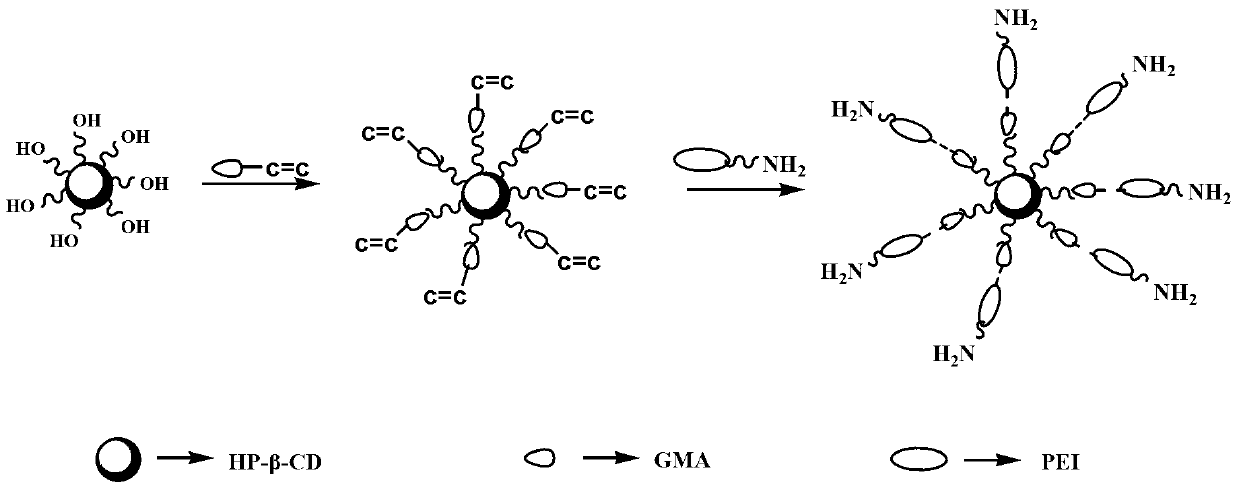
The invention discloses a macromolecule skin penetration enhancer as well as a preparation method and applications thereof. The preparation method comprises the followings step of: linking HP-beta-CD (hydroxypropyl-beta-cyclodextrin) with PEI (polyethyleneimine) by utilizing GMA (glycidyl methacrylate), thereby synthesizing the novel skin penetration enhancer, namely HP-beta-CD-GMA-PEI. Through HP-beta-CD-GMA-PEI treatment, cuticle protein cell viability is improved, so that a keratin accumulation structure becomes loose, and the degree of freedom of the corresponding carbon motion is improved, so that the keratin carbon motion is accelerated. Under the same concentration, compared with azone, the HP-beta-CD-GMA-PEI has a better skin penetration effect, and when the concentration is increased by one time, the skin penetration effect is better.
Owner:武汉天时维璟微生物科技有限公司
Strychnine-hydroxypropyl-beta-cyclodextrin liposome, preparation method and applications thereof
InactiveCN103222956AProlong the action timeIncrease packing capacityOrganic active ingredientsAntipyreticSide effectHalf-life
The present invention discloses a strychnine-hydroxypropyl-beta-cyclodextrin liposome, which contains, by weight, 0.5-2 parts of strychnine, 2-20 parts of a strychnine-HP-beta-cyclodextrin inclusion compound, 20-200 parts of soybean lecithin, and 5-20 parts of cholesterol. The present invention further discloses a strychnine-hydroxypropyl-beta-cyclodextrin liposome preparation method, which comprises: carrying out inclusion on strychnine with hydroxypropyl-beta-cyclodextrin, and then preparing into the liposome. According to the present invention, drug loading capacity is improved, an elimination half-life of the strychnine is prolonged, stability is improved, lymphatic system targeting is achieved, toxic and side effects on heart and central nervous system are reduced, and bioavailability is improved.
Owner:SOUTH CHINA UNIV OF TECH
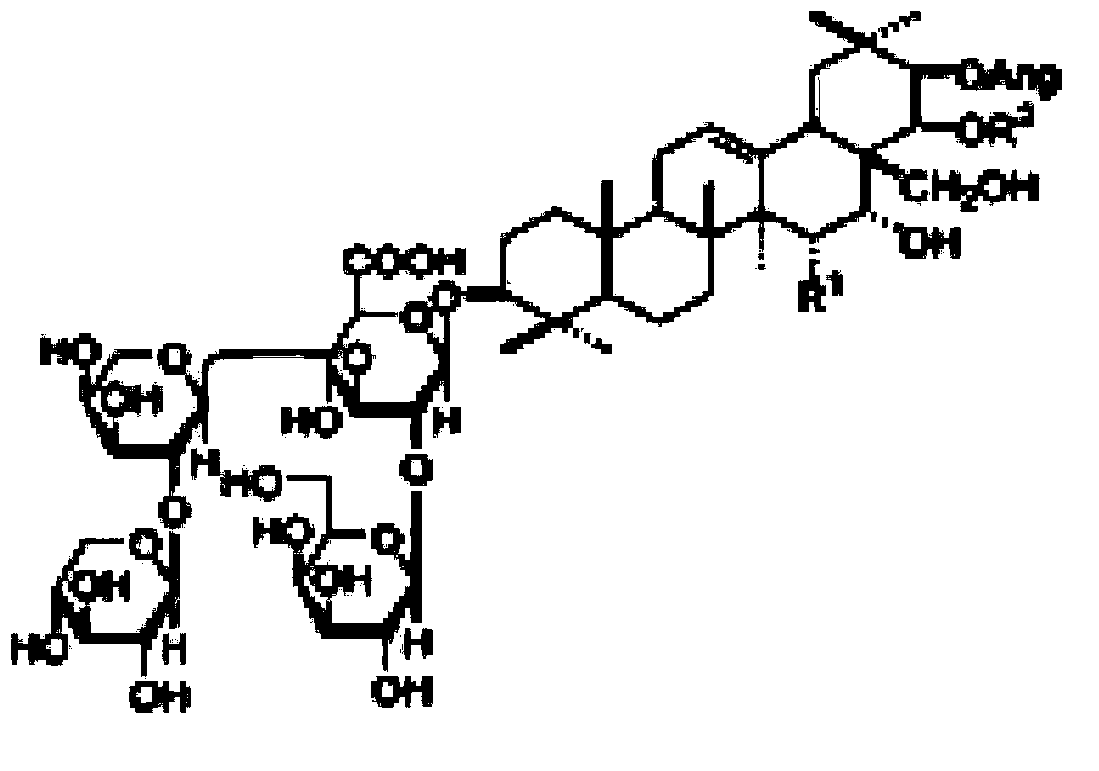
Once preparation method of composite of 17-hydrogen-9-dehydro-andrographolidume-3-sodium (or potassium) disulfate and 17-hydrogen-9-dehydro-andrographolidume-19-sodium (or potassium) disulfate, and purposes of medicine prepared by the same
InactiveCN103159708AEasy to prepareImprove productivityAntibacterial agentsOrganic active ingredientsHydrogenPotassium
The invention relates to a composite of 17-hydrogen-9-dehydro-andrographolidume-3-sodium ( or potassium) disulfate and 17-hydrogen-9-dehydro-andrographolidume-19-sodium (or potassium) disulfate, and discloses components of the composite, and a once preparation method of the components. The composite can be used for preparing medicine having antipyretic, anti-inflammatory and antiviral purposes. The composite is made into dosage forms which can be accepted pharmaceutically.
Owner:JIANGZI QINGFENG PHARMACEUTICALS INC
Medicinal composition containing cefpodoxime proxetil cyclodextrin inclusion compound and preparation thereof
InactiveCN101264087AGood water solubilityImprove solubilityAntibacterial agentsOrganic active ingredientsSolubilityHemolysis
The invention provides a medicine composition comprising the cefpodoxime proxetil cyclodextrin inclusion compound. The basic composition comprises the cefpodoxime proxetil and the pharmacy-acceptable cyclodextrin; the cyclodextrin is chosen from one or a plurality of Beta -cyclodextrin, sulfobutyl- Beta -cyclodextrin, hydroxypropyl- Beta -cyclodextrin or hydroxypropyl-sulfobutyl- Beta -cyclodextrin. The invention has the advantages of increasing the solubility and stability of the medicine, meanwhile lower hemolysis irritability and higher activity. The invention also provides the preparation method of the medicine composition.
Owner:NANJING NORMAL UNIVERSITY +1
Medicine composition containing cefateram cyclodextrin capsule and its preparing method
InactiveCN100998595BLittle hemolysisPreparation method is stableAntibacterial agentsOrganic active ingredientsSolubilityMedicine
A composite medicine with high solubility, stability and activity and low hemolytic irritation contains cefteram ester and the pharmacologically acceptable dextrin chosen from beta-cyclodextrin and its derivatives. Its preparing process is also disclosed.
Owner:海南和德通医药科技信息咨询有限公司
Method for producing camellia oil cake feed through mixed strain fermentation
InactiveCN103039710BPromote growth and reproductionLittle hemolysisAnimal feeding stuffChemistryGenus Lactobacillus
The invention relates to a method for producing camellia oil cake feed through mixed strain fermentation. Bread lactobacillus and bacillus natto are inoculated into camellia oil cake and are subjected to aerobic fermentation and anaerobic fermentation to produce low-toxicity feed. The method provided by the invention overcomes the shortcomings of camellia oil cake in feed application, degrades tea saponin and reduces the hemolysis thereof, degrades cellulose in camellia oil cake, produces flavor substance through lactobacillus fementation and improves the palatability of the feed; and moreover, since bacillus natto is a subspecies of bacillus subtilis, the bacillus natto after heating and drying still exists in the fermented camellia oil cake in a form of spores and thus can realize the function of probiotics.
Owner:SHANGHAI JIAOTONG UNIV +1
Metronidazole composition freeze-dried disintegrating tablets for vaginas and preparation method thereof
ActiveCN102784120BDisintegrates quicklyPromote dissolutionAntibacterial agentsOrganic active ingredientsIrritationFreeze-drying
The invention provides metronidazole composition freeze-dried disintegrating tablets for vaginas and a preparation method of the freeze-dried disintegrating tablets, relates to the field of a medicine and a medicine preparation method technology and mainly solves the technical problems that the metronidazole suppositories and effervescent tablets are inconvenient to transport and store, the application is limited; and the inflamed mucosas are frequently stimulated by the metronidazole suppositories and effervescent tablets when metronidazole suppositories and effervescent tablets are utilized, and the clinical application is influenced. The metronidazole composition freeze-dried disintegrating tablets are prepared from the following components by weight: 10-24% of metronidazole, 24-50% of mannitol, 2-4% of gelatin, 32-74% of hydroxypropyl-beta-cyclodextrin with medium substitution degree and 50% of purified water. The metronidazole composition freeze-dried disintegrating tablets for vaginas prepared by the above components are easy in preparation process, and simple in components, moreover, the metronidazole composition freeze-dried disintegrating tablets for vaginas have the advantages of being fast to disintegrate, convenient to take, and good in compliance, and the tablets take effect quickly and are fully absorbed; and the disintegrating tablets can be completely disintegrated in the vaginas while meeting water, so that foreign body sensation does not exist in the vaginas.
Owner:HAINAN WEI KANG PHARMA QIANSHAN
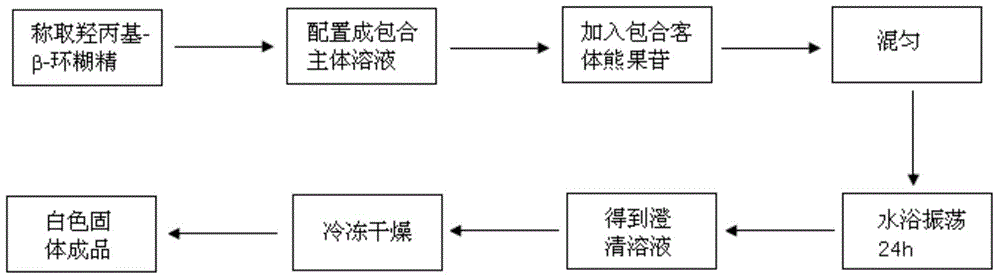
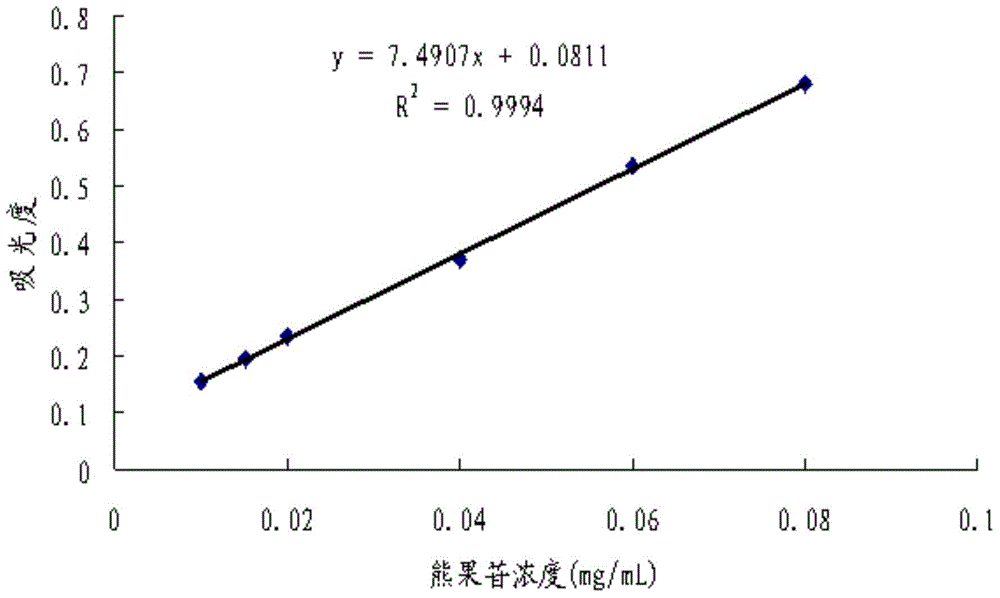
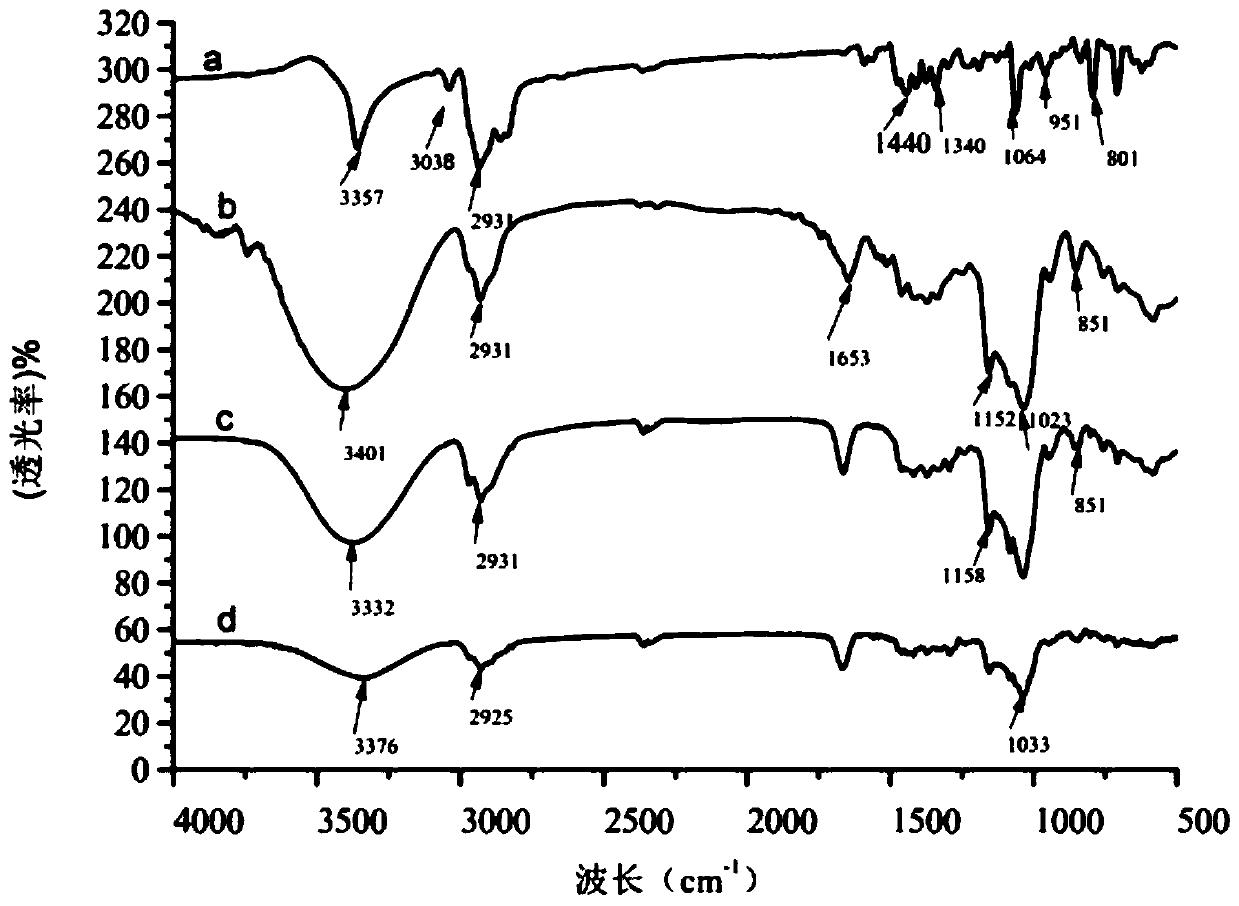
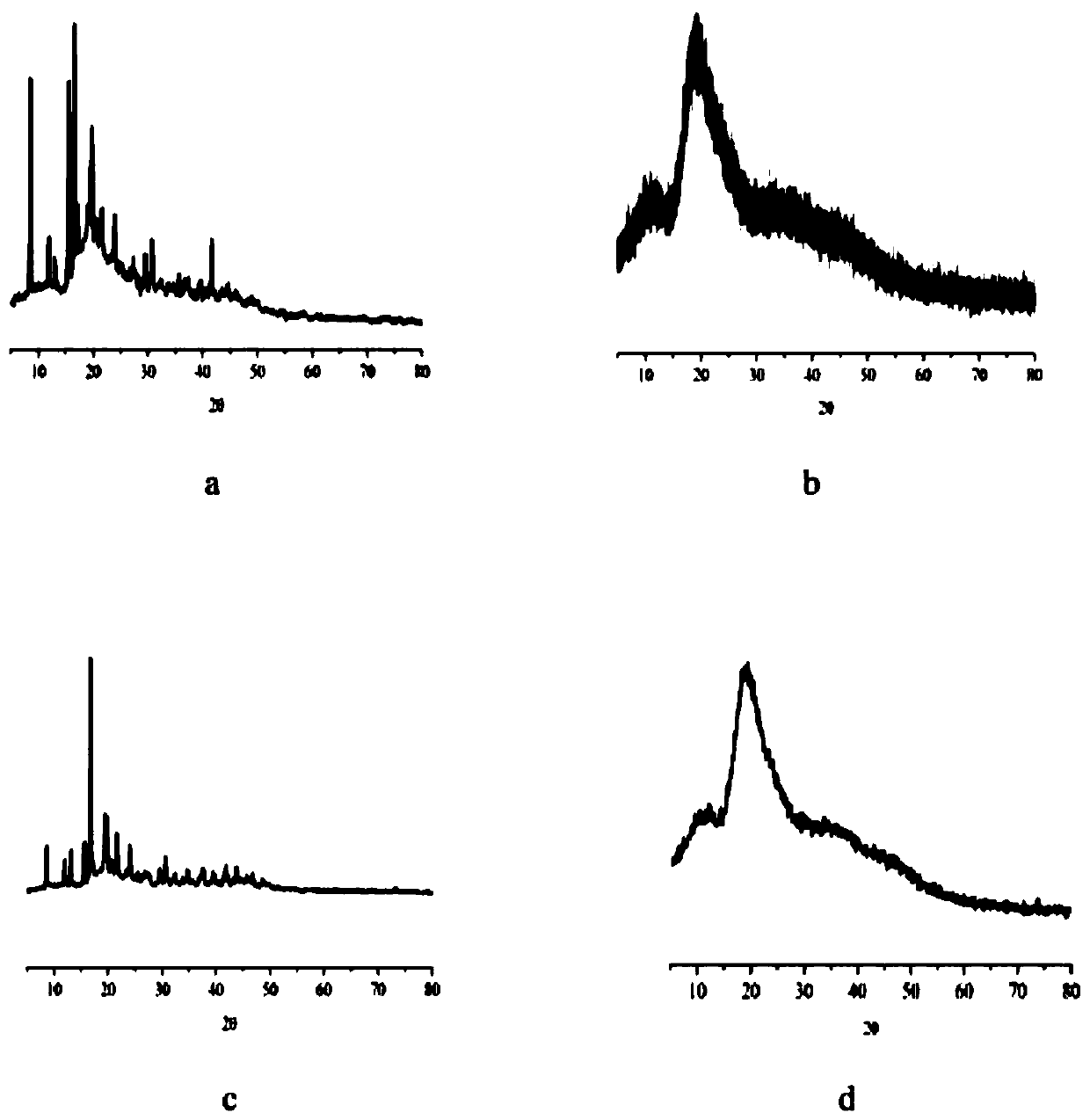
Arbutin/hydroxypropyl-beta-cyclodextrin inclusion compound and preparation method thereof
ActiveCN104622707AImprove thermal stabilityGood water solubilityCosmetic preparationsToilet preparationsArbutinMass ratio
The invention discloses an arbutin / hydroxypropyl-beta-cyclodextrin inclusion compound and a preparation method thereof. The arbutin / hydroxypropyl-beta-cyclodextrin inclusion compound is prepared by inclusion of hydroxypropyl-beta-cyclodextrin and arbutin at the mass ratio of (2:1) to (2:3). According to the arbutin / hydroxypropyl-beta-cyclodextrin inclusion compound prepared by the method disclosed by the invention, the phase of the arbutin is greatly changed; the arbutin exists in the inclusion compound in an amorphous state, and is completely dispersed into the hydroxypropyl-beta-cyclodextrin; the water solubility of the arbutin is increased; the arbutin is combined with the hydroxypropyl-beta-cyclodextrin in the inclusion process in a non-covalent bond form; and the heat stability of the arbutin is significantly improved, so that the application range of the arbutin in cosmetics industry is expanded.
Owner:WUHAN POLYTECHNIC UNIVERSITY
Abiraterone inclusion compound tablet and preparation method thereof
ActiveCN110141556AAccurate doseQuality improvementOrganic active ingredientsPharmaceutical non-active ingredientsSolubilityAdhesive
The invention discloses an abiraterone inclusion compound tablet and a preparation method thereof, and belongs to the technical field of pharmaceutical preparations. The tablet contains an abirateroneinclusion compound and medicinal auxiliary materials. The preparation method comprises the following steps: sieving the abiraterone inclusion compound, a diluent and a disintegrant with a 80-mesh sieve, and performing uniform mixing by an equal-amount increment method; adding an adhesive to prepare a soft material, sieving the obtained soft material with a 14-mesh sieve, preparing wet particles,putting the wet particles into a 60 DEG C oven, performing drying until the moisture content is 1-2%, performing sieving with a 16-mesh sieve, and weighing the dry particles; and adding a lubricant which is 1% by weight of the dry particles, and performing uniform mixing and tabletting. The method prepares the abiraterone inclusion compound into a tablet. The tablet has the advantages of accuratedosage, stable quality, low cost, convenient carrying and the like, is a preferred dosage form of the pharmaceutical preparation, and solves and improves the technical problems of compressibility andsolubility of the abiraterone tablet.
Owner:李建恒
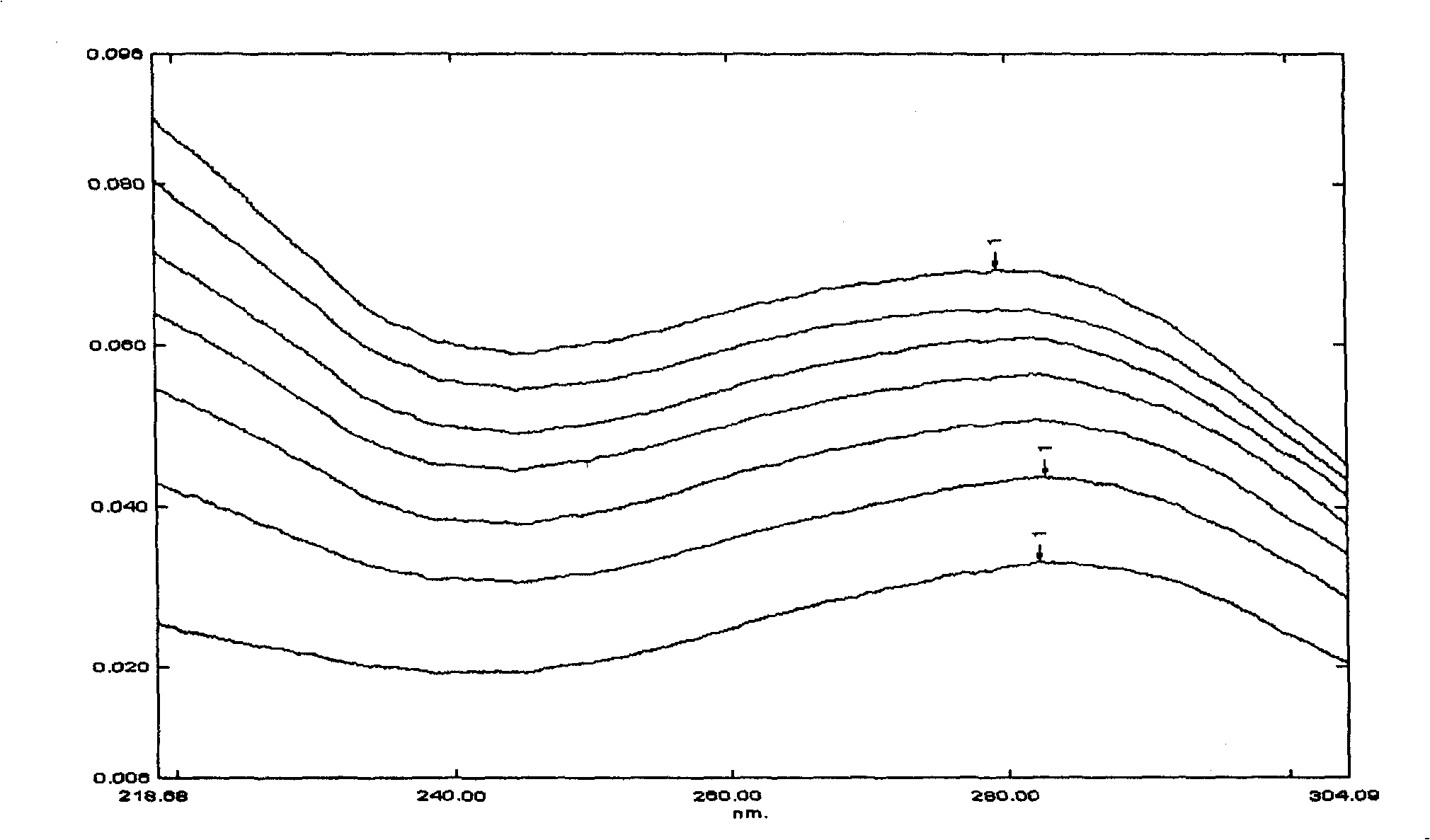
Application of the sulfur-butyl ether-belta-cyclodextrin in eyedrops of chloramphenicol and method thereof
InactiveCN101549160AFast dissolutionBlock hydrolysis reactionOrganic active ingredientsSenses disorderMethyl aldehydeSulfur
The present invention is application of the sulfur-butyl ether-belta-cyclodextrin in eyedrops of chloramphenicol and method thereof. Adding sulfur-butyl ether -belta-cyclodextrin into formula of the eyedrops of chloramphenicol, the addition is 10-80 g / L. The invention finds SBE-belta-CD (sulfur-butyl ether -belta-cyclodextrin) for improving stability of the eyedrops of chloramphenicol by a series of experiments, capable of improving dissolvability of the chloramphenicol for six times, and because of inclusion of the cavity structure to chloramphenicol, blocking chloramphenicol hydrolysis reaction catalyzed by various ions in solution effectively, degradation of the chloramphenicol in aqueous solution is greatly relieved, by experiments, accelerating for six months and normal temperature storing for 24 months, every index of the chloramphenicol eyedrops still conforms to request of the standard, content of chloramphenicol glycol is less than 8%, content of methyl aldehyde is 0, so stability of the chloramphenicol eyedrops is greatly improved.
Owner:昆明振华制药厂有限公司
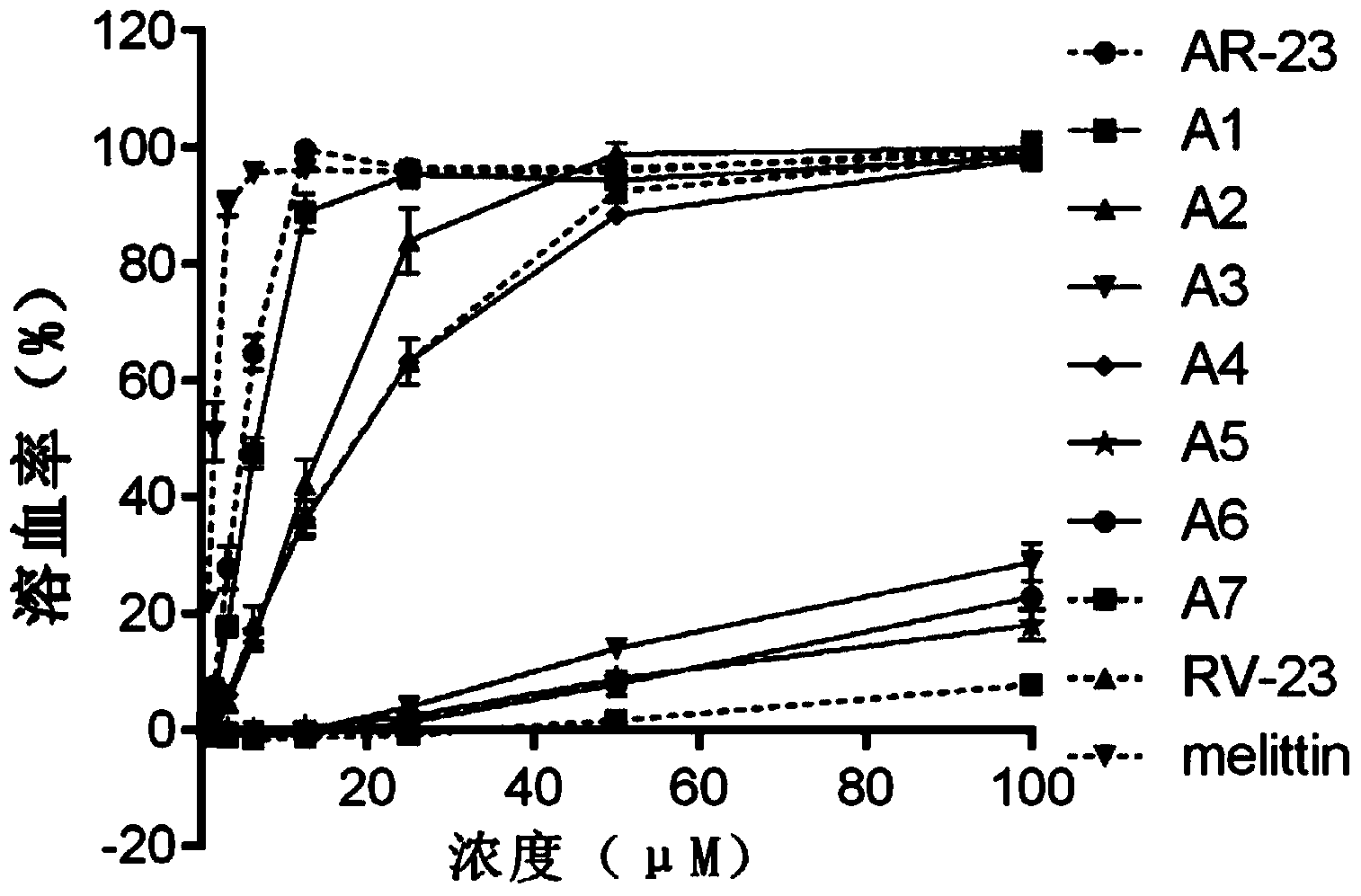
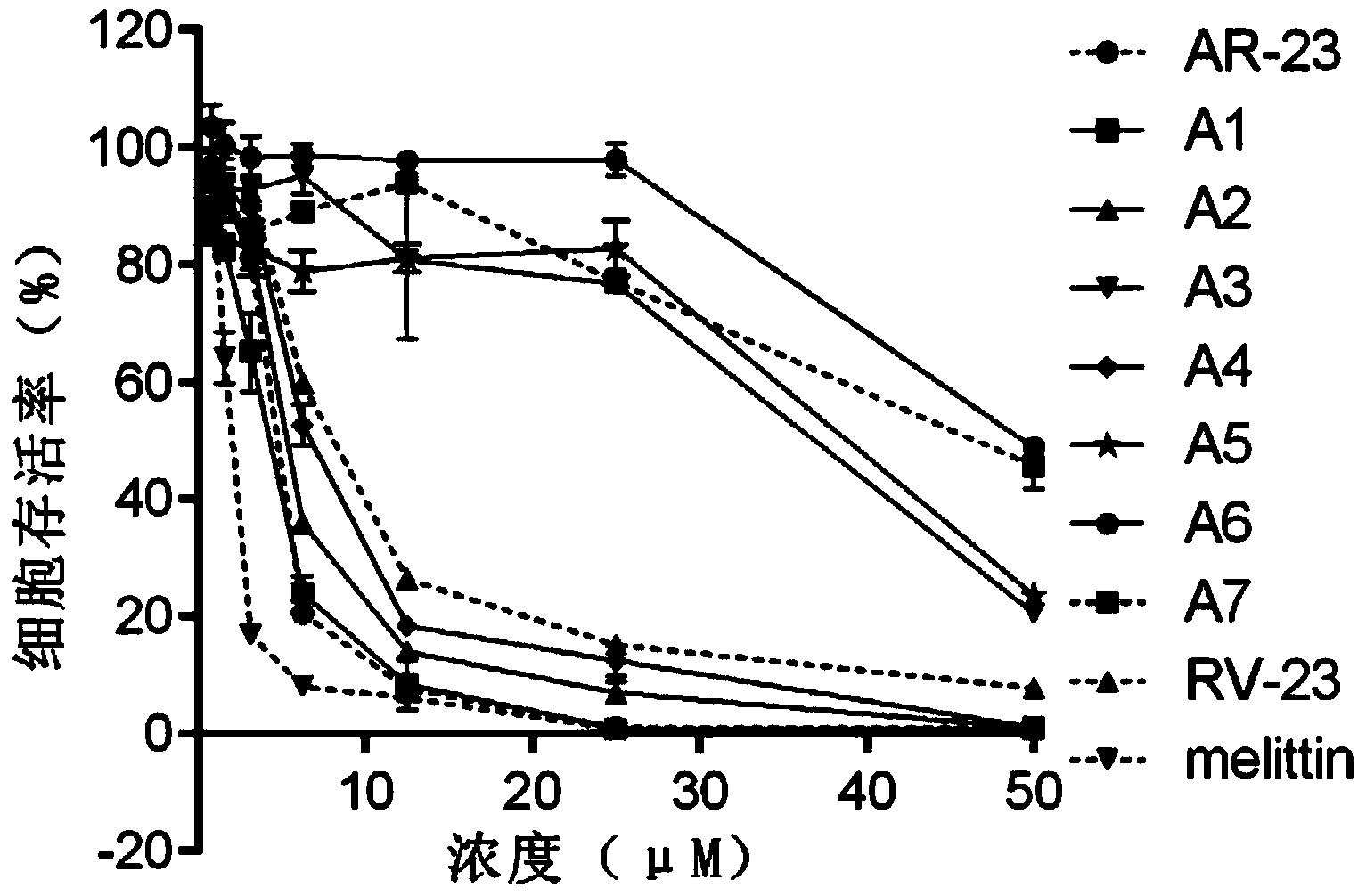
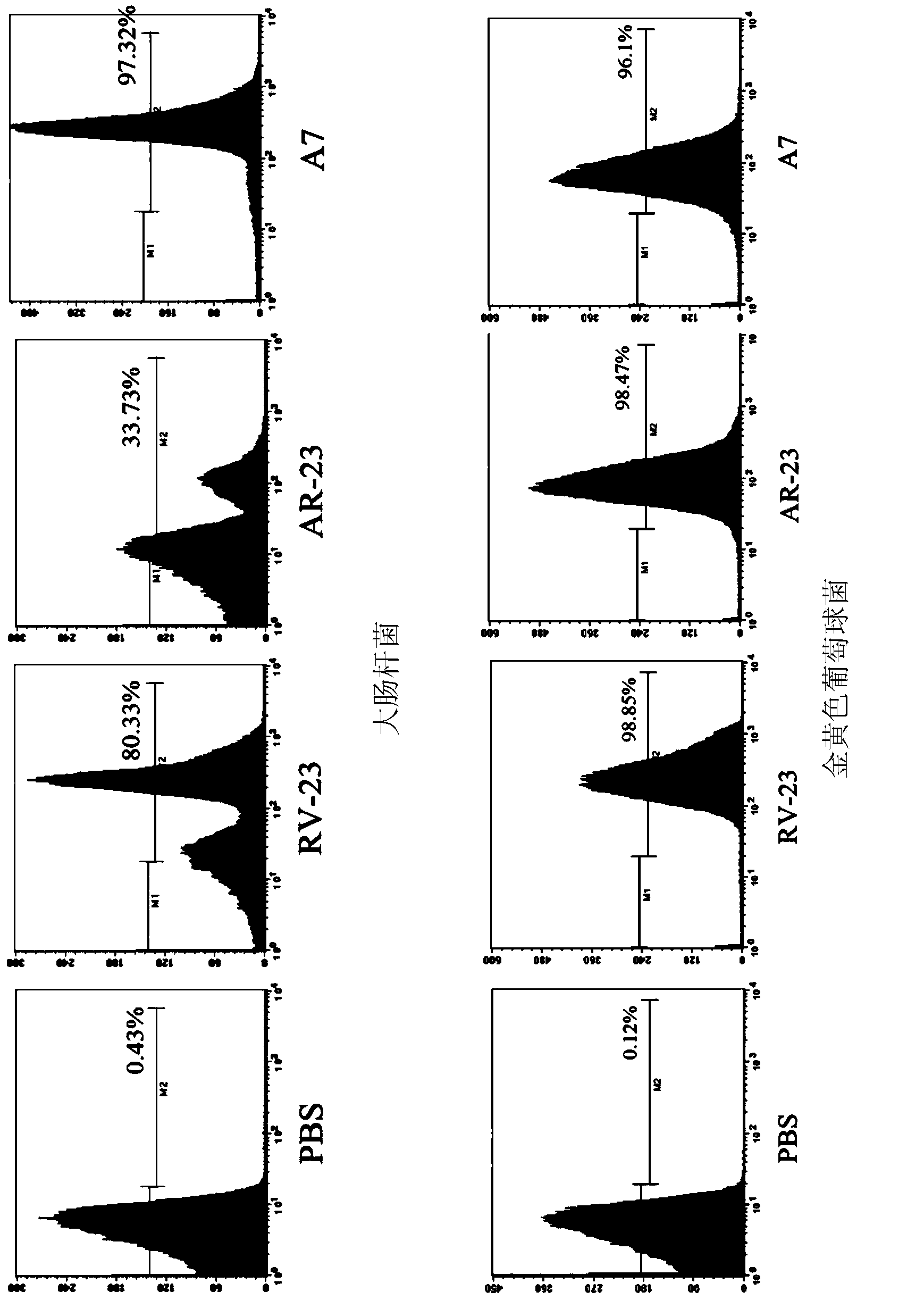
Derived polypeptides formed by modifying structures of frog skin antibacterial peptides AR-23 and application of derived polypeptides
InactiveCN104109198ALow toxicityReduced dissolution abilityAntibacterial agentsPeptide/protein ingredientsBacteroidesRed blood cell
The invention discloses a group of derived polypeptides formed by modifying the structures of frog skin antibacterial peptides AR-23 and an application of the derived polypeptides. The group of derived polypeptides are named as A1-A7, and have amino acid sequences, i.e., respectively SEQ ID No.1, SEQ ID No.2, SEQ ID No.3, SEQ ID No.4, SEQ ID No.5, SEQ ID No.6 and SEQ ID No.7. The experiment proves that compared with natural antibacterial peptides, according to the derived polypeptides formed by modifying the structures of frog skin antibacterial peptides AR-23, the selective toxicity of the derived polypeptides to bacteria is high while the toxicity of the derived polypeptides to human cells is obviously decreased, so that the influences of the derived polypeptides to somatic cells, red blood cells and the like are very low. The polypeptides A1-A7 have broad-spectrum killing effects on gram-positive bacteria or gram-negative bacteria, so that the polypeptides A1-A7 can be used for treating diseases caused by the infections of the gram-positive bacteria or the gram-negative bacteria resistant to antibiotics.
Owner:FIELD OPERATION BLOOD TRANSFUSION INST OF PLA SCI ACAD OF MILITARY
PH-responsive amphotericin B derivative as well as preparation method and application thereof
ActiveCN113603738AReduce nephrotoxicityLittle hemolysisOrganic active ingredientsAntimycoticsAntifungalHemolysis
The invention relates to a pH-responsive amphotericin B derivative as well as a preparation method and application thereof, and belongs to the technical field of pharmaceutical chemistry preparation. According to the derivative of the invention, the amphotericin B derivative with a novel structure is obtained by modifying amino on trehalosamine in an amphotericin B molecule, and compared with non-modified amphotericin B, the amphotericin B derivative has the characteristics of low renal toxicity, small hemolysis and high safety. In addition, the invention further provides a preparation method of the derivative, the yield is high as well, and the prepared derivative has a good antifungal effect and has a wide market prospect in the aspect of treating systemic deep fungal infection.
Owner:SOUTHWEST UNIVERSITY
Clarified propofol injection and preparation method thereof
InactiveCN111150703ALow free propofol concentrationImprove stabilityHydroxy compound active ingredientsInorganic non-active ingredientsPropofol InjectionCyclodextrin
The invention relates to clarified propofol injection and a preparation method thereof. The injection includes propofol, cyclodextrin, a stabilizing agent, a pH regulating agent and injection water; the pH value of the injection is 6-9, and preferably is 7-8.5. The injection can be filtered online, and is few in ingredient kinds, low in using amount, low in free propofol concentration, convenientin storage and good in stability.
Owner:BIKA BIOTECH GUANGZHOU CO LTD
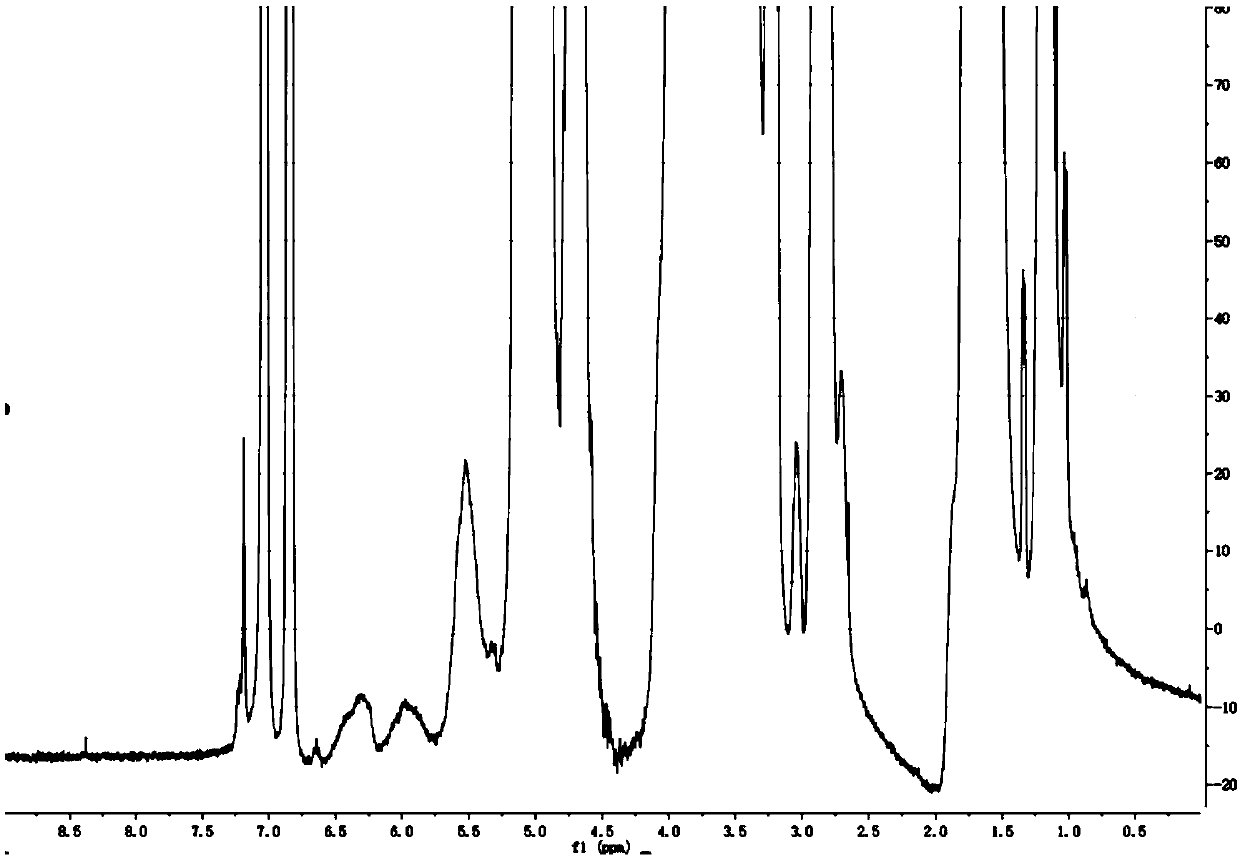
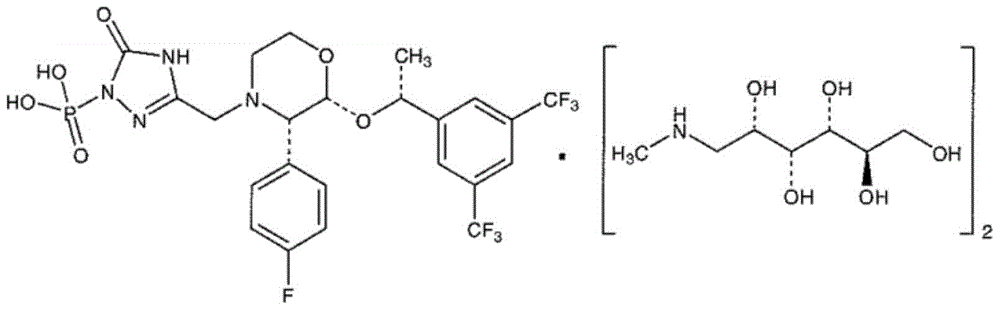
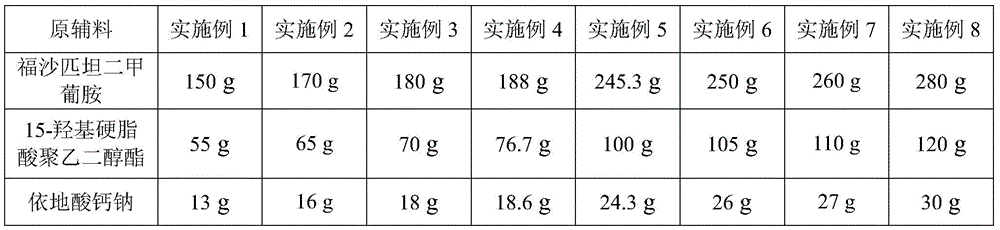
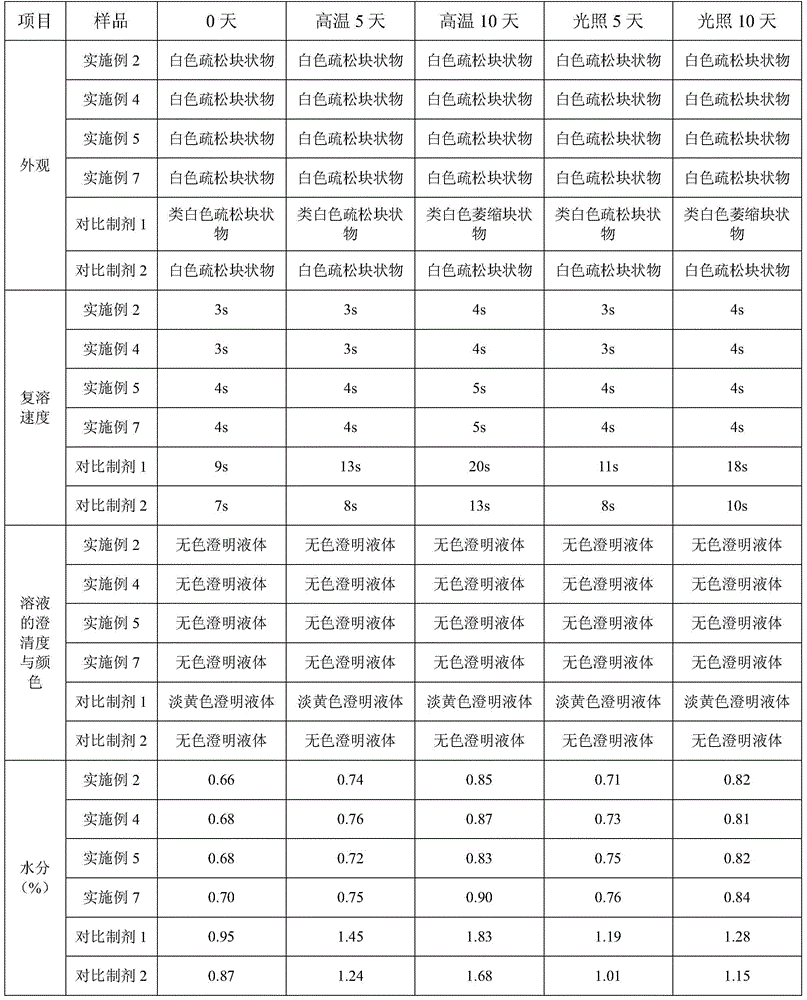
A kind of fosaprepitant dimeglumine composition for injection and preparation method thereof
ActiveCN104414980BEliminate security risksImprove securityOrganic active ingredientsPowder deliveryHydroxystearic AcidPolyethylene glycol
The invention relates to a fosaprepitant dimeglumine composition for injection and a preparation method thereof, the pharmaceutical composition is composed of fosaprepitant dimeglumine, 15-polyethylene glycol hydroxystearate and sodium calcium edentate, prescription is simple, technology is simple, and the prepared product has the advantages of stable quality, safety and reliability.
Owner:CHENGDU EASTON BIOPHARMACEUTICALS CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com