Once preparation method of composite of 17-hydrogen-9-dehydro-andrographolidume-3-sodium (or potassium) disulfate and 17-hydrogen-9-dehydro-andrographolidume-19-sodium (or potassium) disulfate, and purposes of medicine prepared by the same
A technology of andrographolide, sodium sulfate, applied in 17-hydro-9-dehydroandrographolide-3-sulfate sodium (or potassium), 17-hydro-9-dehydroandrographolide-19- The one-time preparation of the sodium sulfate (or potassium) composition and the field of its pharmaceutical use can solve the problems of difficulty in preparing liquid preparations, restricting production efficiency, complicated production processes, etc., to ensure pharmacological activity, the preparation method is simple and convenient, and the water solubility is good. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
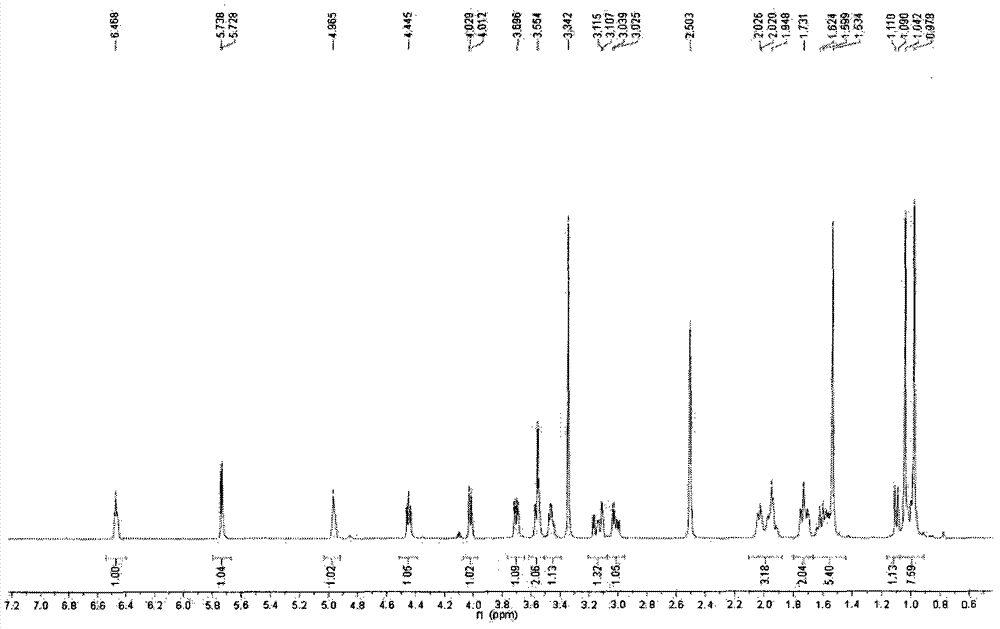
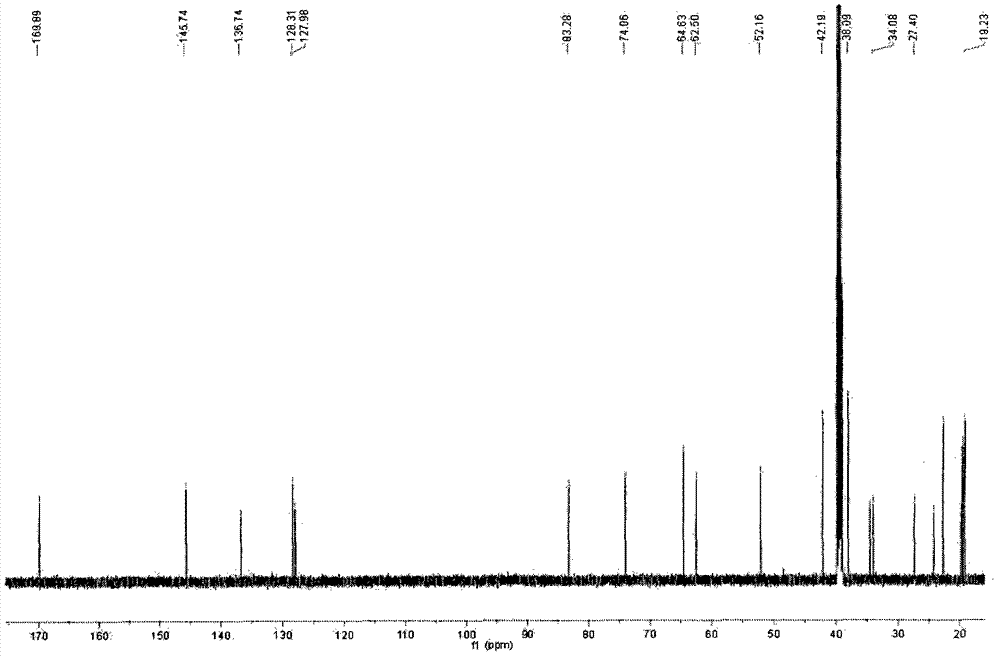
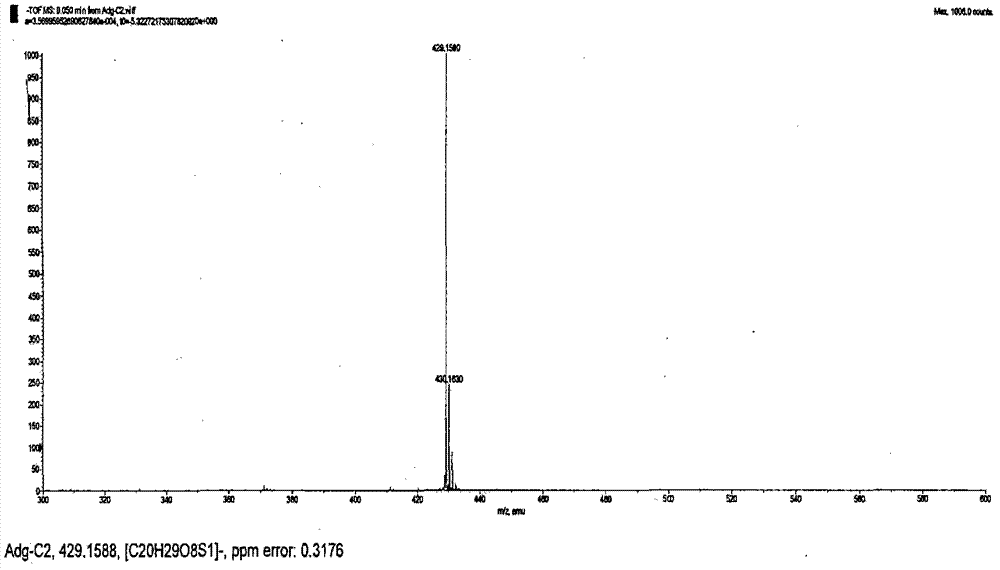
[0063] Take andrographolide, add 5.5 times the amount of acetic anhydride (62%) and glacial acetic acid (38%) to dissolve, while stirring, slowly add 4.5 times the amount of sulfuric acid at a rate of 1.6ml per minute of 1g andrographolide (52%) and glacial acetic acid (48%), mix well, adjust the temperature of the reactor at 16°C, and place it for 90 minutes to sulfonate. Add the same amount of purified water, stir well, adjust the pH to about 7.0 with 33% NaOH, add 95% ethanol to make the alcohol content more than 82%, recover the ethanol under reduced pressure, and separate the aqueous solution by macroporous adsorption resin and ODS column chromatography. Elute with a gradient of water:ethanol, the ratio of water is 80-20%, and the ratio of ethanol is 20-80%. Collect the eluate in sections, combine the same components, and crystallize to obtain 17-hydro-9-dehydroandrographis Sodium lactone-3-sulfate, its sodium salt molecular formula C 20 H 29 NaO 8 S, molecular weight: 452...
Embodiment 2
[0071] Take andrographolide, add 5.6 times the amount of acetic anhydride (60%) and glacial acetic acid (40%) to dissolve it, while stirring, slowly add 4.5 times the same amount of sulfuric acid at a rate of 2.0 ml per minute of 1g andrographolide Mix well with glacial acetic acid, adjust the temperature of the reactor at 18°C, and let it stand for 100 minutes to sulfonate. Add the same amount of purified water, stir well, adjust the pH to about 7.0 with 36% NaOH, add 95% ethanol to make the alcohol content more than 82%, recover the ethanol under reduced pressure, and separate the aqueous solution by macroporous adsorption resin and ODS column chromatography. Elute with a gradient of water: ethanol, the ratio of water is 80-20%, the ratio of ethanol is 20-80%, collect the eluent components, combine the same components, and crystallize to obtain 17-hydro-9-dehydroandrographis Sodium lactone-19-sulfate, the molecular formula of its sodium salt: C 20 H 29 NaO 8 S, molecular weig...
Embodiment 3
[0079] Take andrographolide, add 5.5 times the amount of acetic anhydride (60%) and glacial acetic acid (40) to dissolve, while stirring, add 3.5 times the amount of sulfuric acid (52%) at a rate of 2.1ml per minute for 1g andrographolide Mix well with glacial acetic acid (48%), adjust the temperature of the reaction kettle at 17°C, place for 80 minutes to sulfonate, and pour the reactants into saturated potassium chloride solution to obtain 17-hydro-9-dehydroandrographolide- 3-potassium sulfate, 17-hydro-9-dehydroandrographolide-19-potassium sulfate and other mixed solutions are concentrated under reduced pressure and dried in vacuum to obtain a mixture. The mixture is dissolved in a small amount of water and passed through a Sephadex LH-20 column. The loading ratio of the mixture to Sephadex LH-20 is 1:7, and the column diameter to height ratio of Sephadex LH-20 is 1:28. Elute with 3 times the column volume of water, discard the eluent, and use 25% ethanol for 6 times the colu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com