Derived polypeptides formed by modifying structures of frog skin antibacterial peptides AR-23 and application of derived polypeptides
A technology of antimicrobial peptides and polypeptides, which is applied in the fields of application, antibacterial drugs, and medical preparations containing active ingredients, etc., which can solve problems such as side effects, decreased hemolytic activity, and impact on the application prospects of antibacterial drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] Embodiment 1, the synthesis of polypeptide A1-A7
[0072] This application reconstructed the sequence of AR-23, and synthesized the modified derivative polypeptides of the frog skin antimicrobial peptide AR-23 named A1-A7, as shown in Table 1, the amino acid sequence of A7 is the SEQ ID No in the sequence table .1. It can be seen from the amino acid sequence that SEQ ID No.2 consists of 23 amino acid residues, and is a derivative polypeptide obtained by mutating Arg at the first and eighth positions of SEQ ID No.1 into Ala respectively. The polypeptide is named A3; SEQ ID No.3 consists of 23 amino acid residues, and is a derivative polypeptide obtained by mutating Arg at position 8 of SEQ ID No.1 to Ala, and the derived polypeptide is named A5; SEQ ID No. .4 consists of 23 amino acid residues, and is a derivative polypeptide obtained by mutating Arg at position 1 of SEQ ID No.1 to Ala, and the derivative polypeptide is named A6; SEQ ID No.5 consists of 23 amino acid re...
Embodiment 2
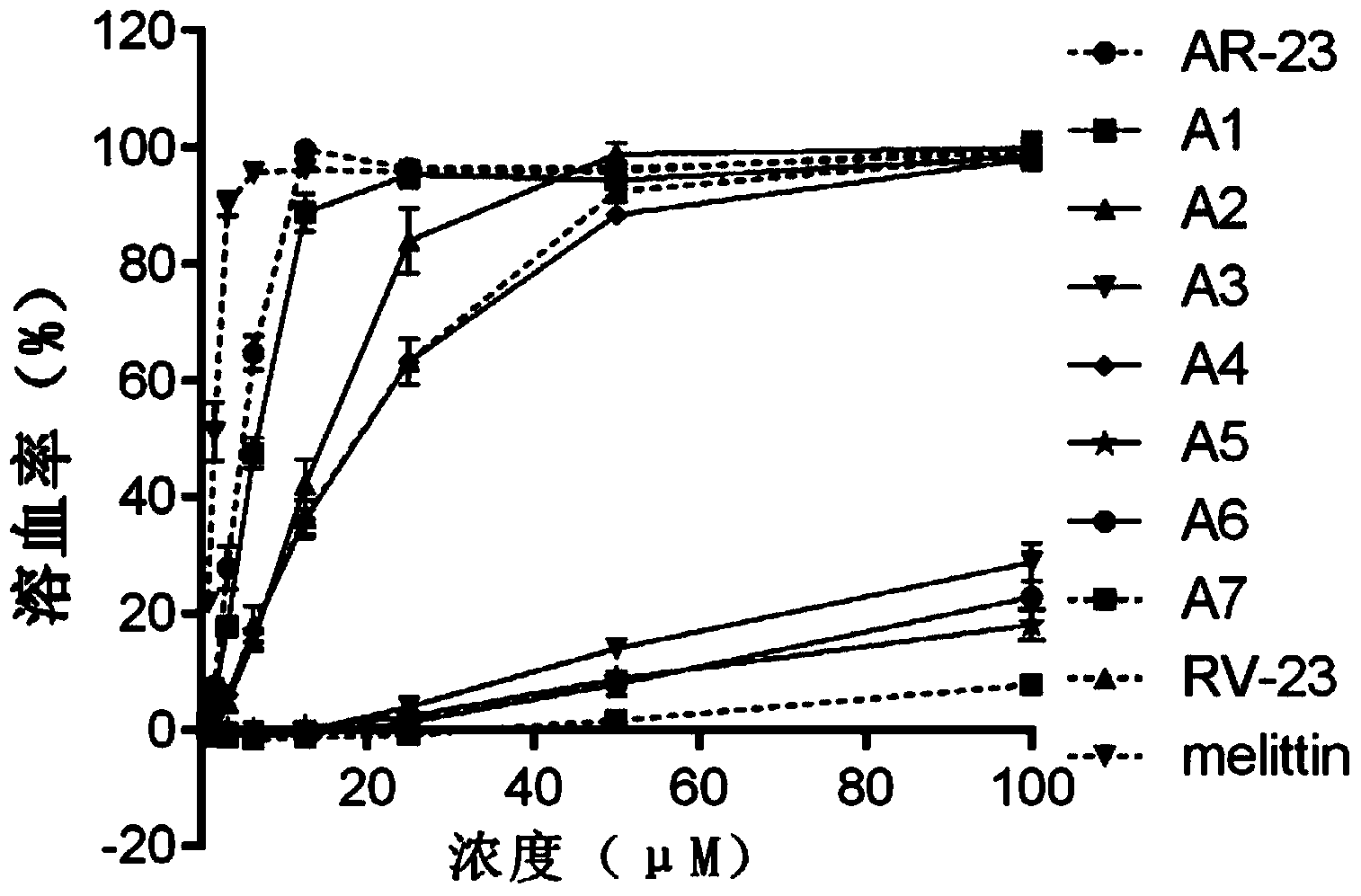
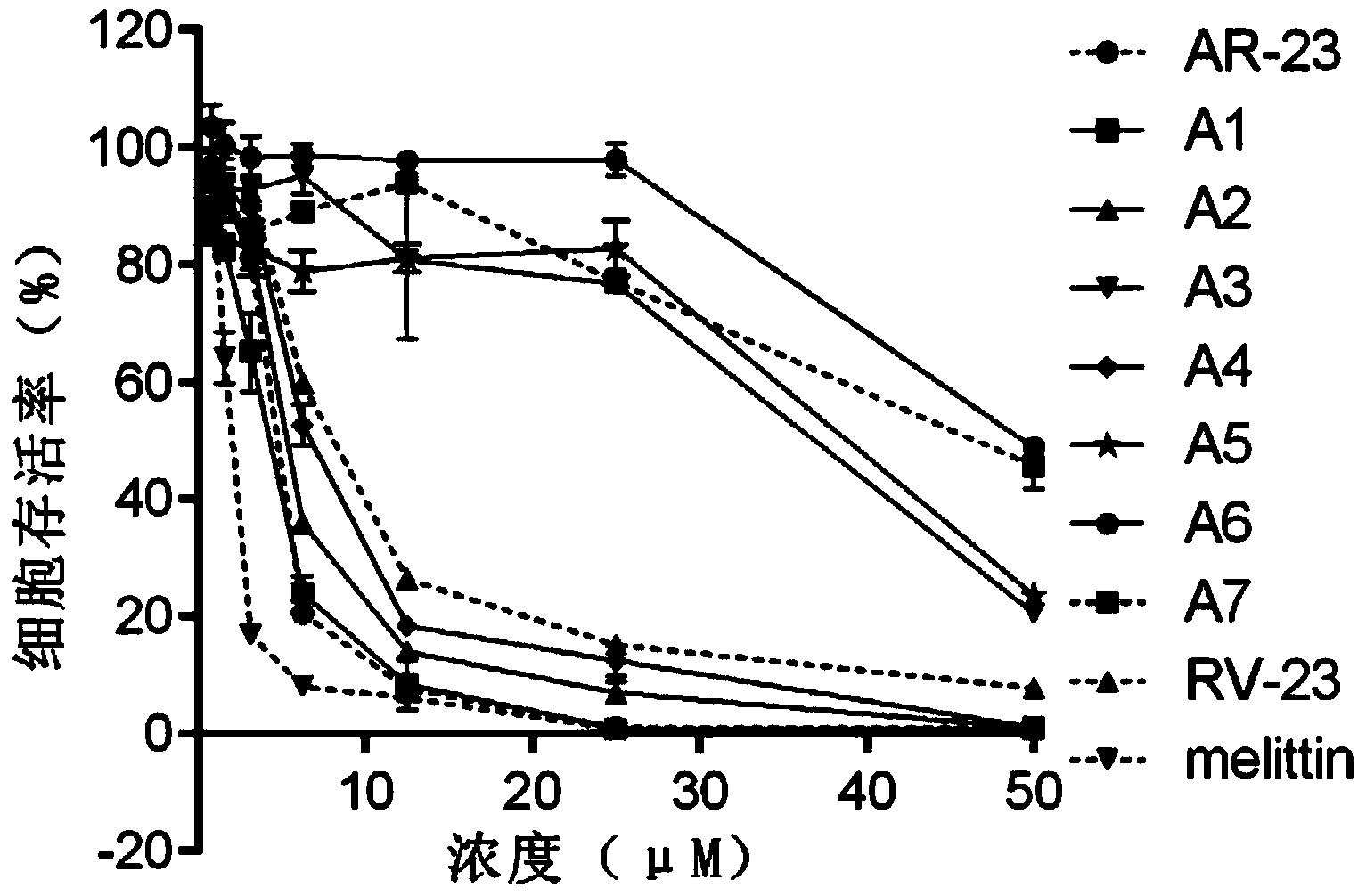
[0077] Example 2. Identification of Polypeptide A1-A7's Ability to Lyse Erythrocytes
[0078] The experiment was repeated three times, and the experimental method for each repetition was as follows:
[0079] Fresh human erythrocytes were washed three times with PBS buffer, and prepared into 1.25% (v / v) erythrocyte suspension with PBS buffer. Dissolve the polypeptides A7, A1, A2, A3, A4, A5 and A6 obtained in Example 1 with different volumes of PBS buffer solution respectively, and make polypeptide A7 solution, A1 solution, A2 solution, A3 solution, A4 solution, A5 solution and A6 solution. At the same time, AR-23, RV-23 and melittin were used as a comparative experiment, and different concentrations of AR-23 solution, RV-23 solution and melittin solution were prepared. Take 40 μL of different concentrations of polypeptide A7 solution, A1 solution, A2 solution, A3 solution, A4 solution, A5 solution, A6 solution, AR-23 solution, RV-23 solution and melittin solution, and add it...
Embodiment 3
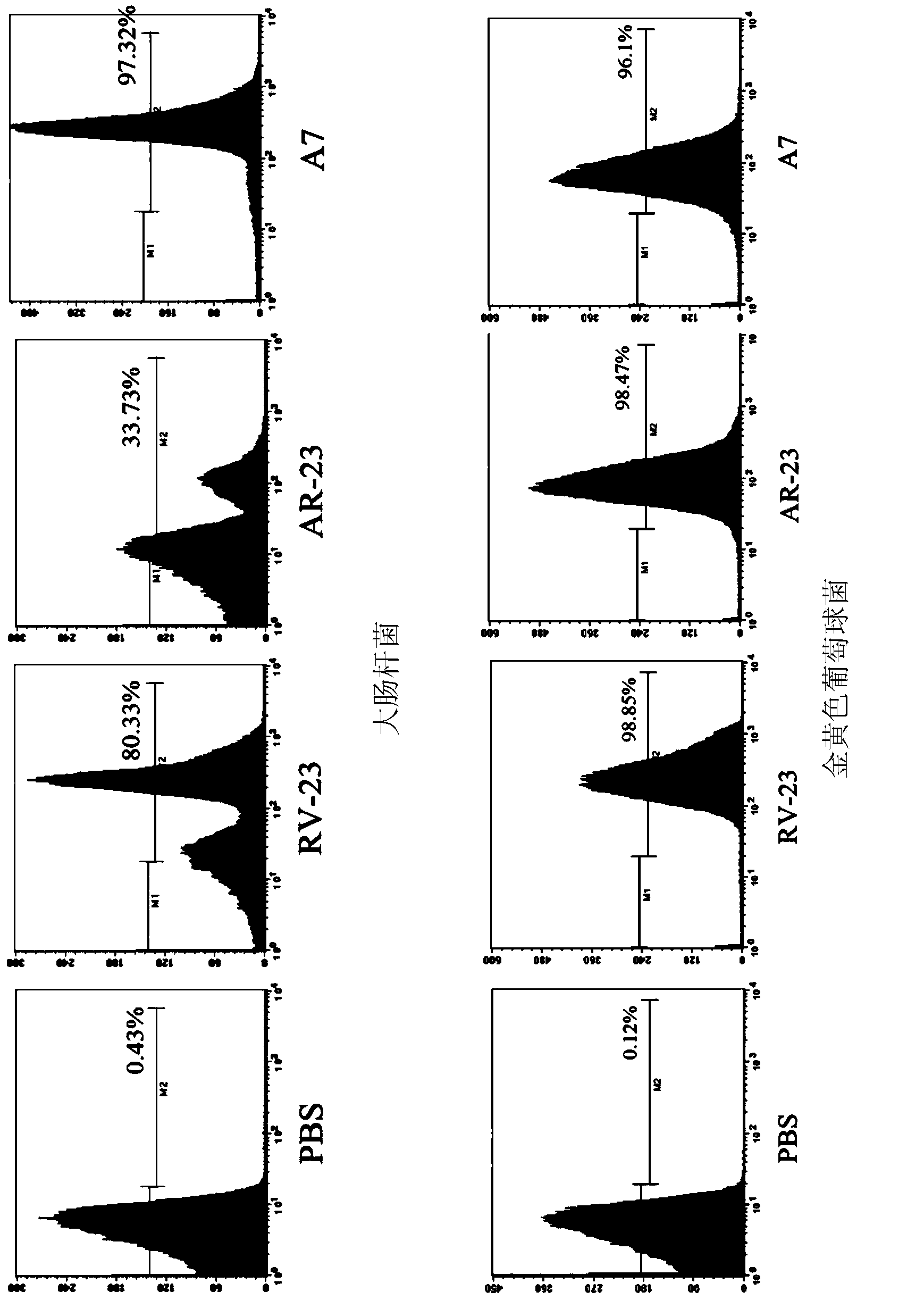
[0085] Embodiment 3, the minimum inhibitory concentration of polypeptide A1-A7 to different bacteria
[0086] The minimum inhibitory concentrations of polypeptides A1-A7 to different bacteria were identified by the micro-dilution method using a 96-well plate. The experiment was repeated three times, and the method of repeating the experiment each time was as follows:
[0087] Escherichia coli (China General Microbiology Collection Center, number: 1.3373), Pseudomonas aeruginosa (China General Microbiology Collection Center, number: 1.2620), Staphylococcus aureus (China General Microbiology Collection Center, number: 1.2910) and Staphylococcus epidermidis (China Center for Common Microorganisms Collection, No. 1.4260) were streaked and inoculated onto LB plates respectively, and spent overnight at 37. Pick single colonies of Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus and Staphylococcus epidermidis respectively in ordinary LB medium, culture at 37 degrees a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com