Patents
Literature
54 results about "Human erythrocytes" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Human Erythrocytes. In an average adult, each cubic millimeter of blood contains more than five million erythrocytes, which are commonly known as red blood cells.
Human erythrocyte membrane antigen coated microsphere and application thereof
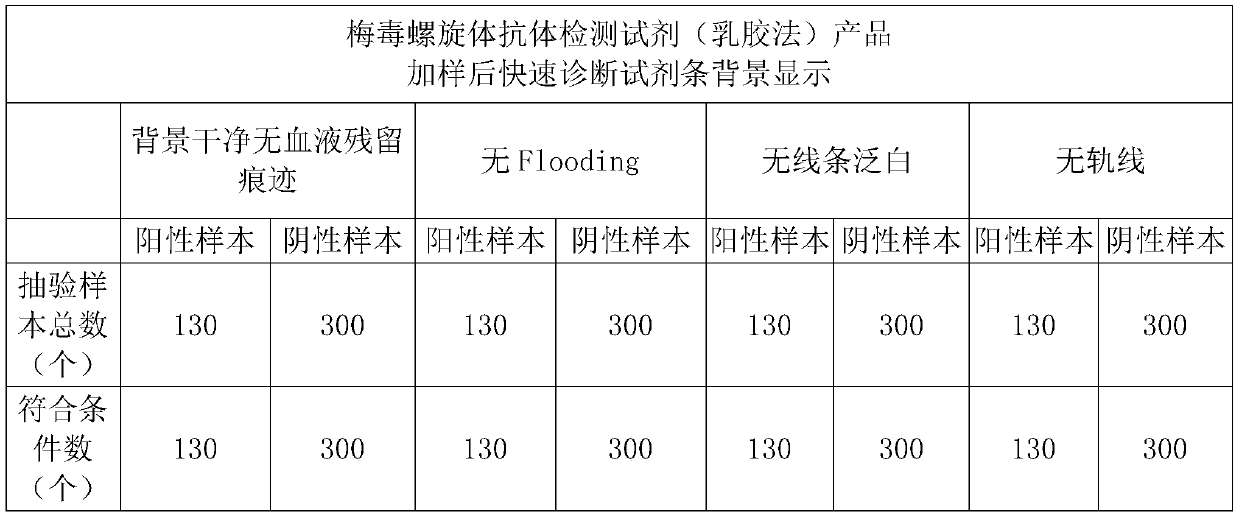
InactiveCN101957366AMaintain and preserve antigenic activityLong validity periodBiological testingAntiendomysial antibodiesMicrosphere
The invention provides a method for preserving the activity of a human cell membrane blood group antigen, which comprises the steps of: preparing an erythrocyte membrane blood group antigen extract, and then coating the prepared erythrocyte membrane blood group antigen on a solid microsphere so as to replace a fresh erythrocyte to be used for detecting a blood group antibody in a sample.
Owner:INTEC PROD INC
Hybridoma cell line and anti-human erythrocyte surface H antigen monoclonal antibodies generated thereof
InactiveCN101037671AImmunoglobulins against cell receptors/antigens/surface-determinantsFused cellsAntigenGene engineering
The invention discloses a hybridization tumor cell stem 2E8Z CGMCC NO.1942 and monoclonal antibody of human red blood cell surface H antigen thereof. The monoclonal antibody 2E8 of human red blood cell surface H antigen genereated by hybridization tumor cell stem has advantages of high effect, strong differentia, extensive distribution of antigen. The flexible peptide is easy to be bended, so mixed albumen of the invnetion is feld correctly and without influence each activity combination area of the mixed albumen. Experiment evidence, the mixed albumen can specially combine with human red blood cell surface H antigen; moreover, the mixed albumen with high expression amount, easy to be purified, short production cycle, large production scale and low cost. Base on these advantages, the invention plans to use gene engineering method to produce double function molecule and establish good base of treating based on red blood cell and testing platform, having more actual sense and wider application foreground in medical testing field.
Owner:FIELD OPERATION BLOOD TRANSFUSION INST OF PLA SCI ACAD OF MILITARY
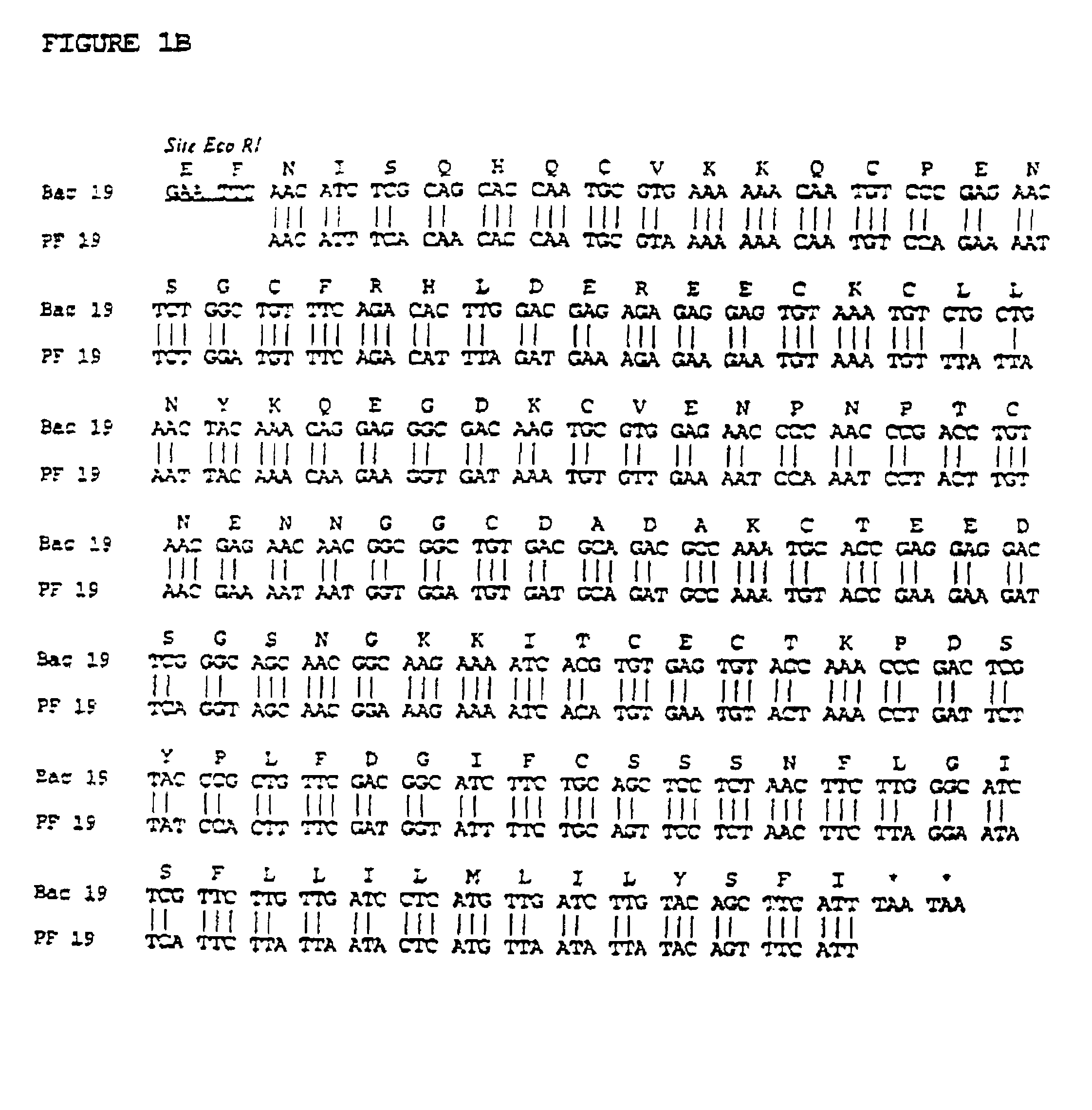
Recombinant protein containing a C-terminal fragment of plasmodium MSP-1
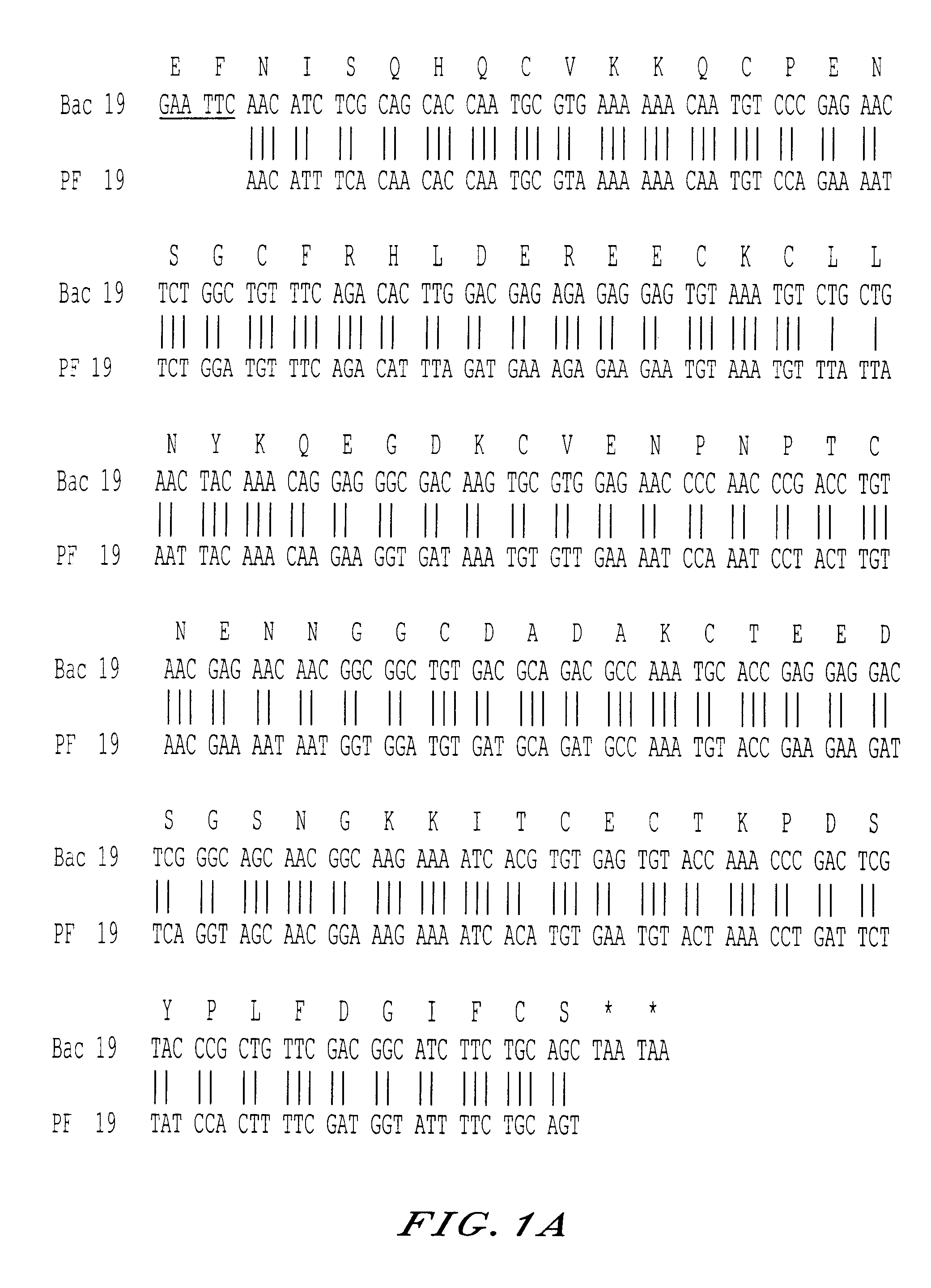
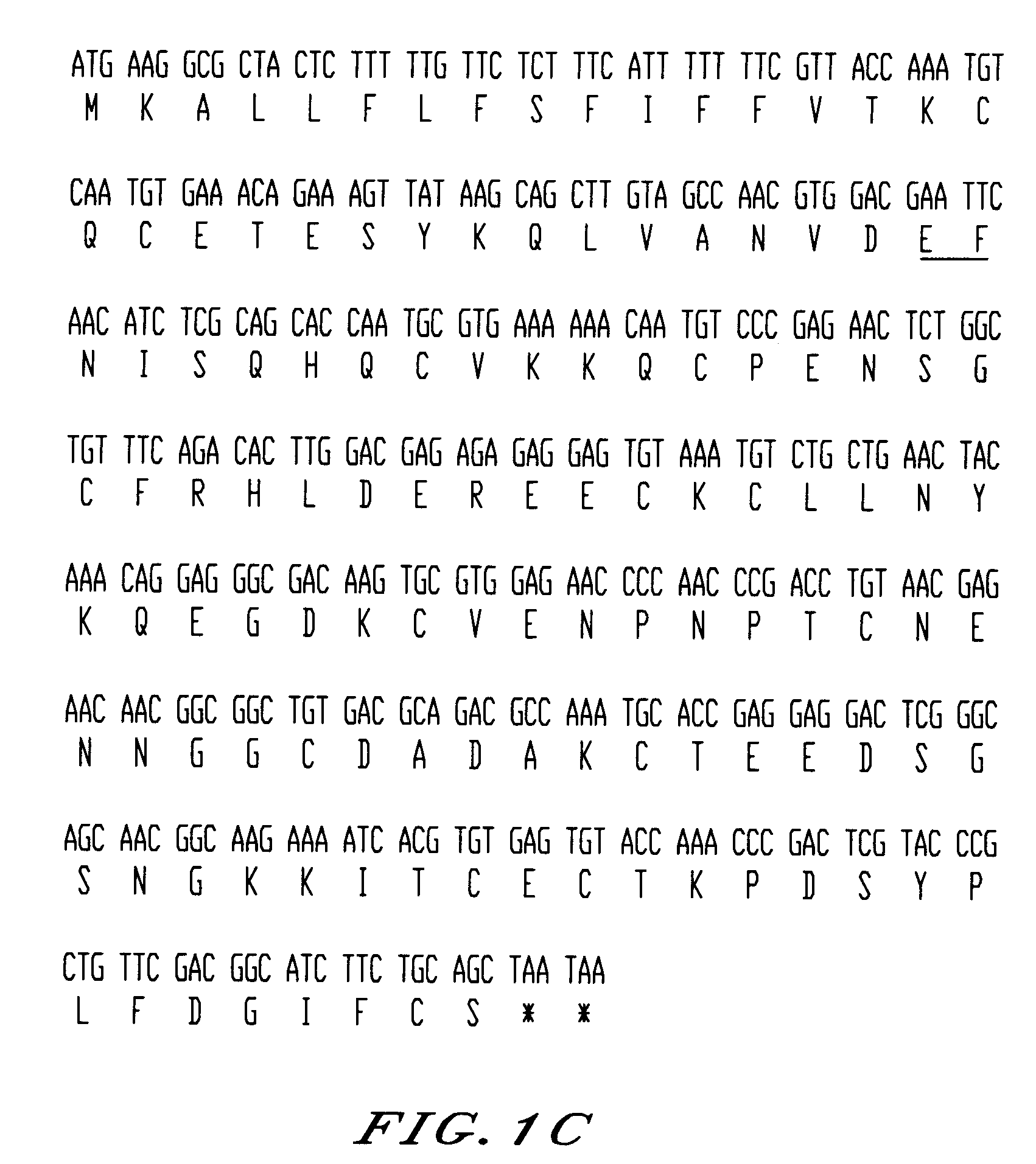
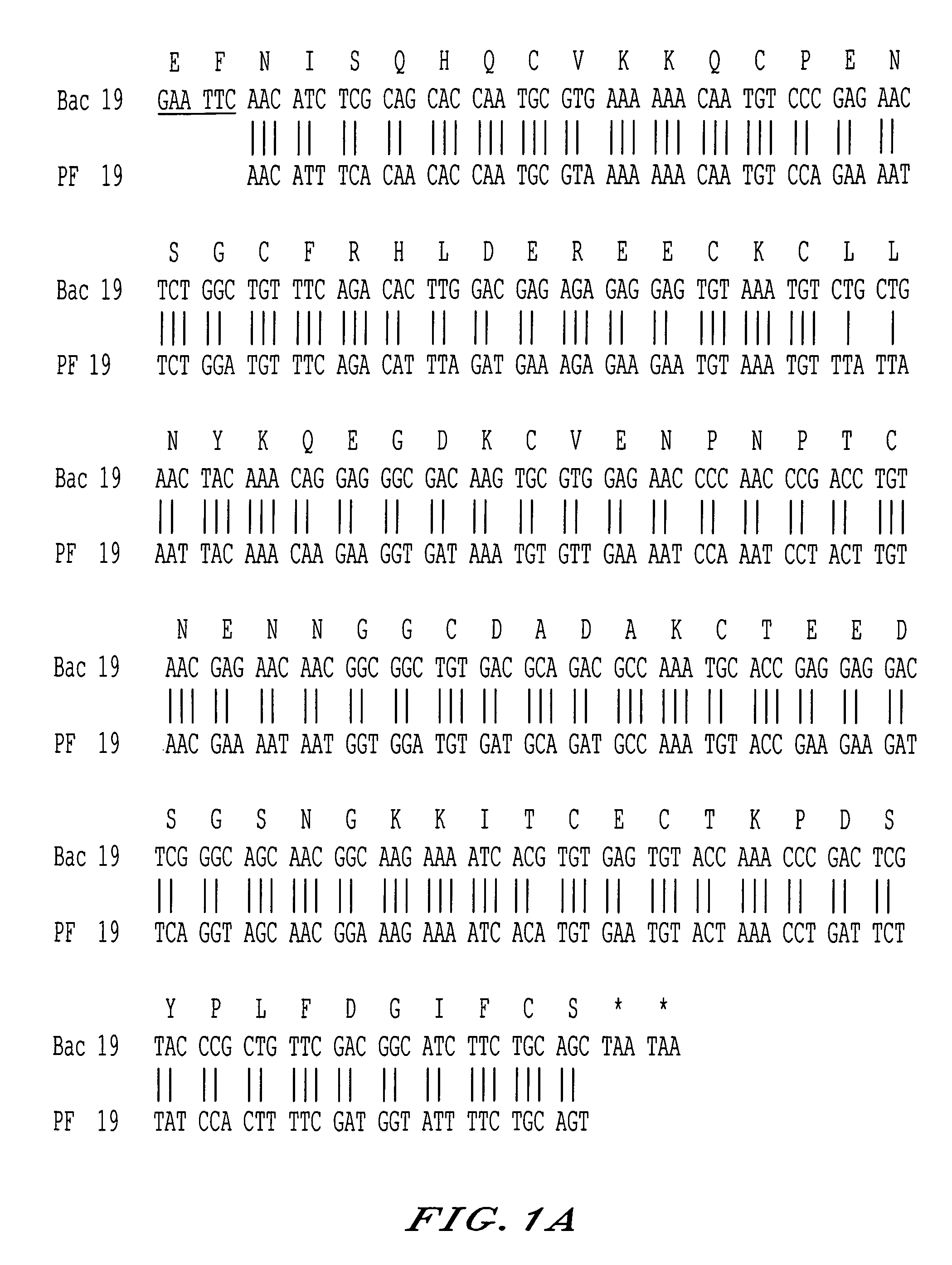
The invention relates to a recombinant protein fabricated in a baculovirus system, of which the essential constitutive polypeptide sequence is that of a C-terminal fragment of 19 kilodalton (p19) of the surface protein 1 (protein MSP-1) of the merozoite parasite of the Plasmodium type, particularly Plasmodium falciparum, which is infectious for humans, said C-terminal fragment remaining normally anchored at the surface of the parasite at the end of its penetration phase into human erythrocytes, in the occurrence of an infectious cycle. Said recombinant protein is applicable to the production of vaccines against malaria.
Owner:INST PASTEUR +1
In vitro mass production of human erythroid cells from the blood of normal donors and thalassemic patients
InactiveUS20040229356A1FavorInhibit productionArtificial cell constructsBlood/immune system cellsGlycophorinThalassemia
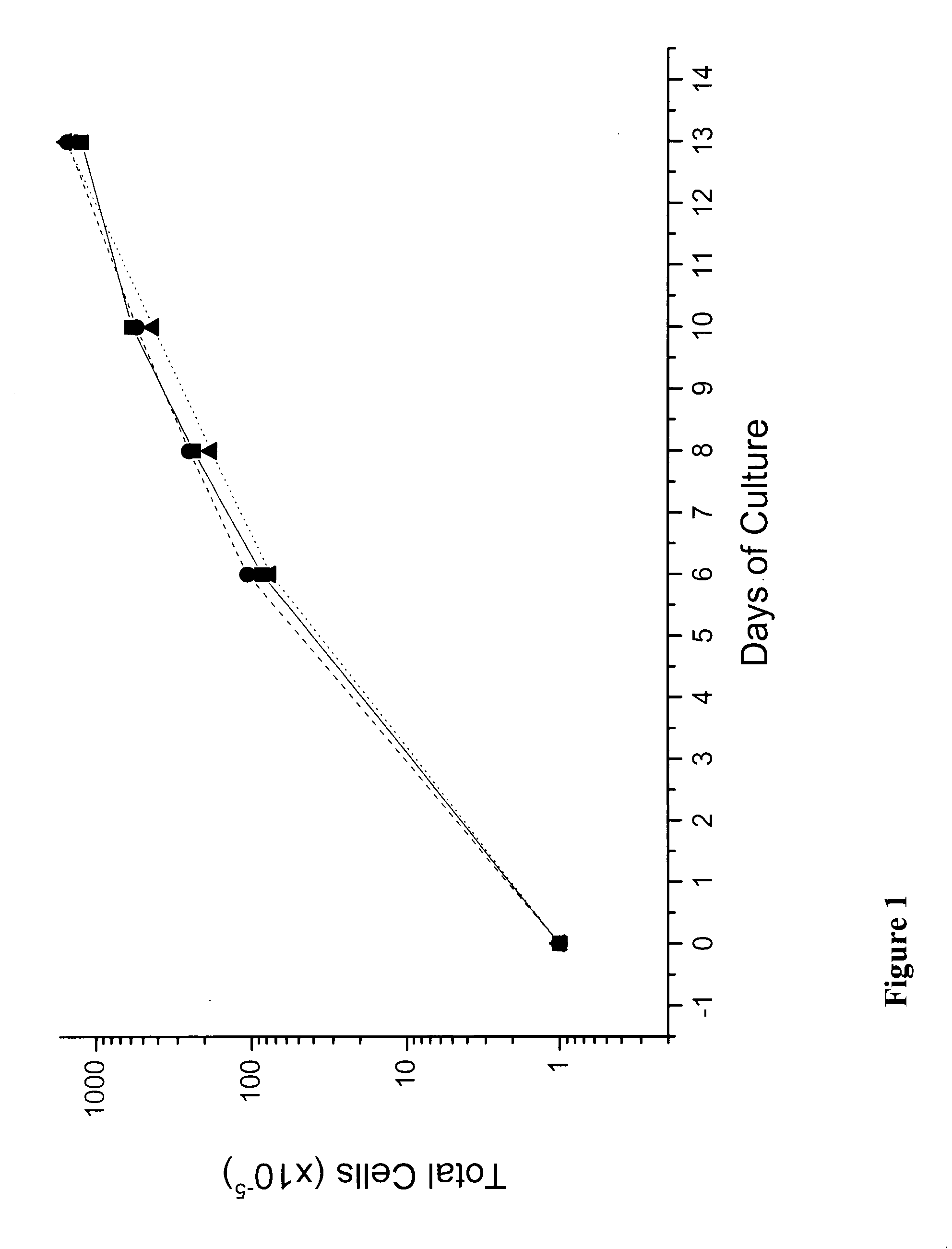
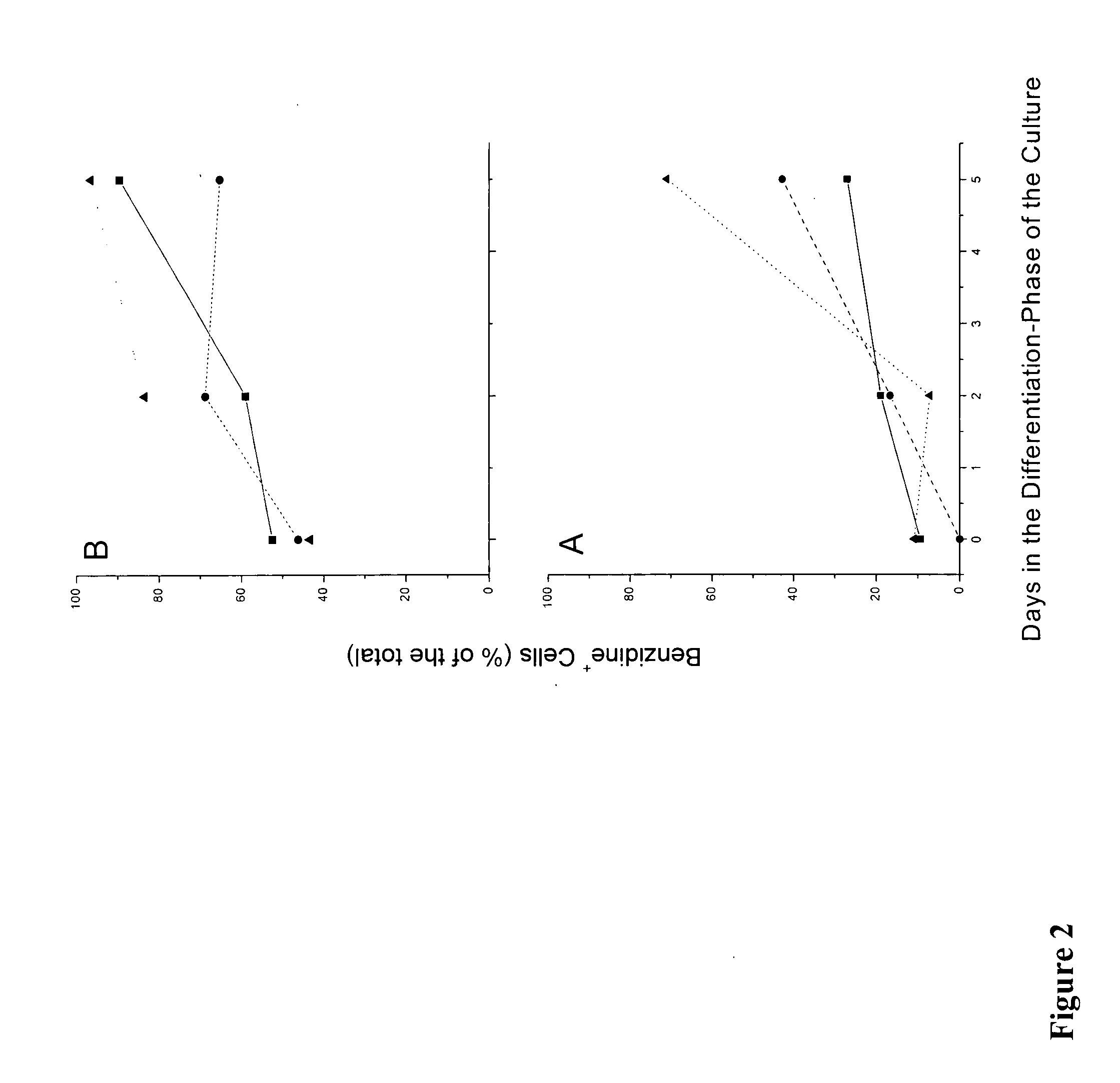
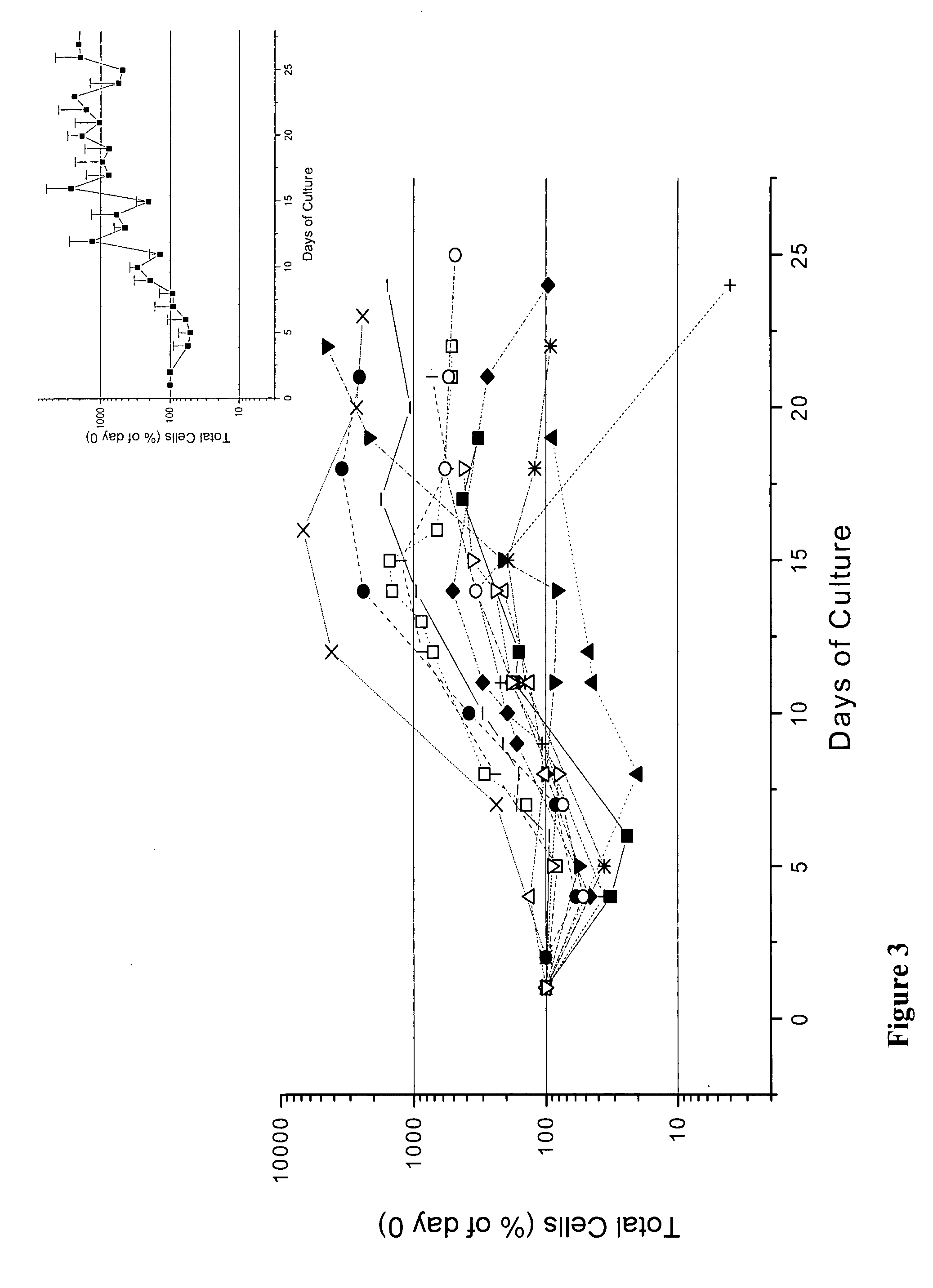
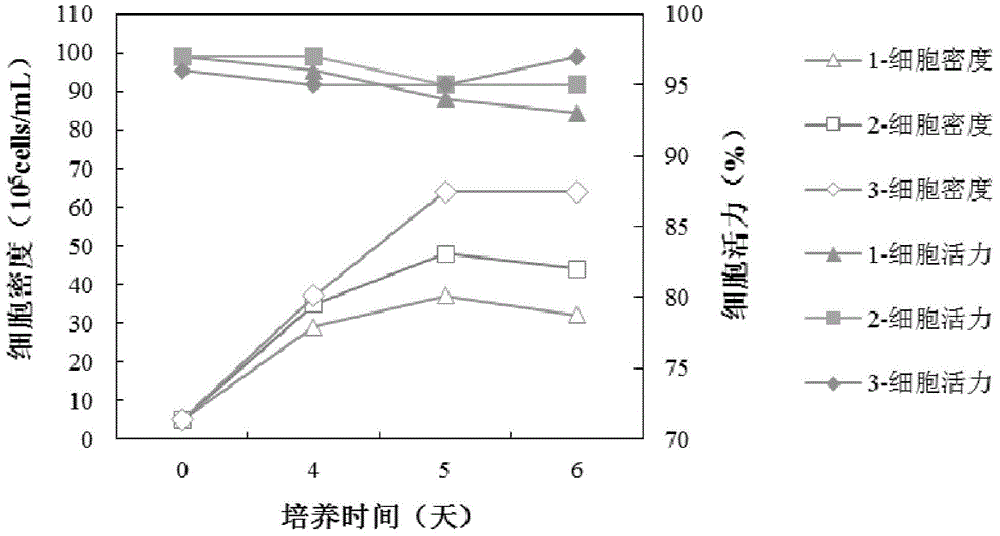
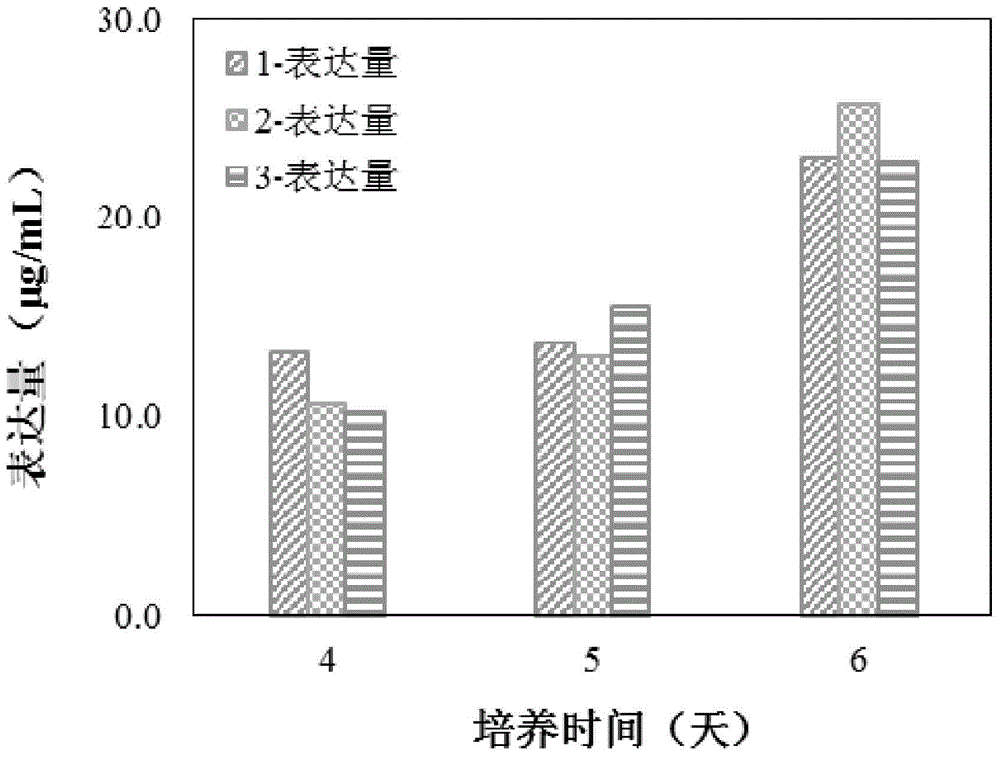
We describe a new two-step culture method for mass production in vitro of erythroid cells from either CD34<+> (10<5 >cells / mL) or light-density (10<6 >cells / mL) cells purified from the blood of normal donors and thalassemic patients. The method includes (i) culture of the cells in the presence of dexamethasone and estradiol (10<-6 >M each) and (ii) the growth factors SCF (50 ng / mL), IL-3 (1 ng / mL), and EPO (1 U / mL). In their proliferative phase, these cultures generated about 1-2x10<7 >erythroblasts for each milliliter of blood collected from normal donors or thalassemic patients. They were composed mostly (90%) of CD45<low> / glycophorin (GPA)<neg> / CD71<low >cells at day 7, 50-60% of which became CD45<neg> / GPA+ / CD71<high >by days 15-20. However, when cells from days 7 to 12 of the proliferative phase were transferred in differentiation medium containing EPO and insulin, they progressed to mature erythroblasts (>90% benzidine<pos >and CD45<neg> / GPA<+> / CD71<medium>) in 4 days. Because of the high number of erythroid cells that are generated from modest volumes of blood, this method will prove useful in donor-specific studies of erythroid differentiation.
Owner:INST SUPERIORE DI SANITA
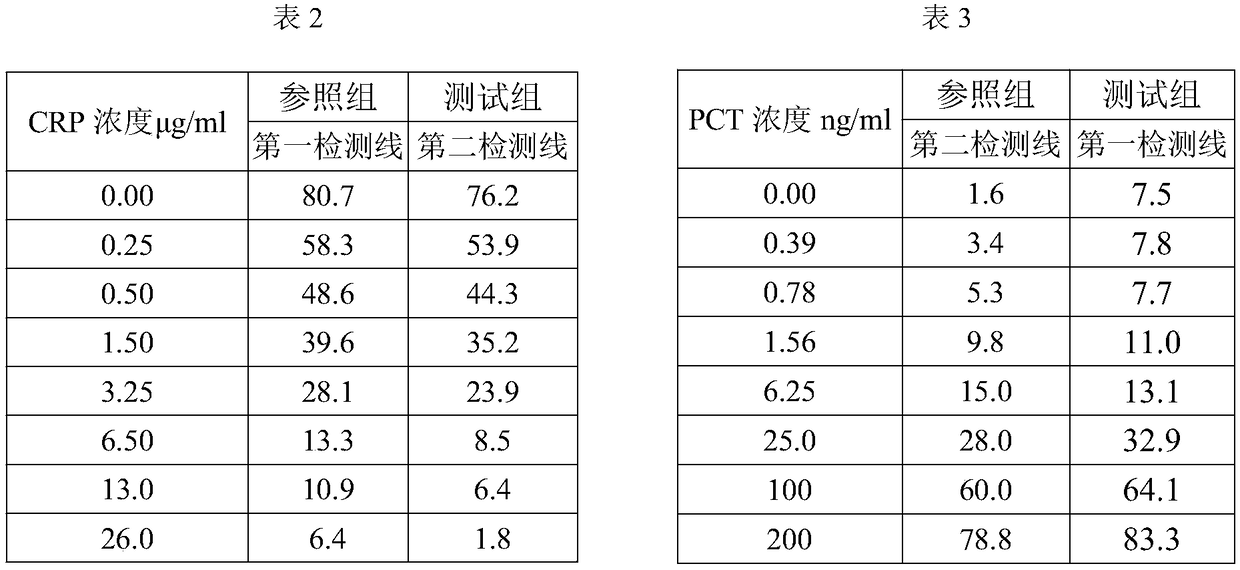
PCT-CRP dual card and preparation method thereof
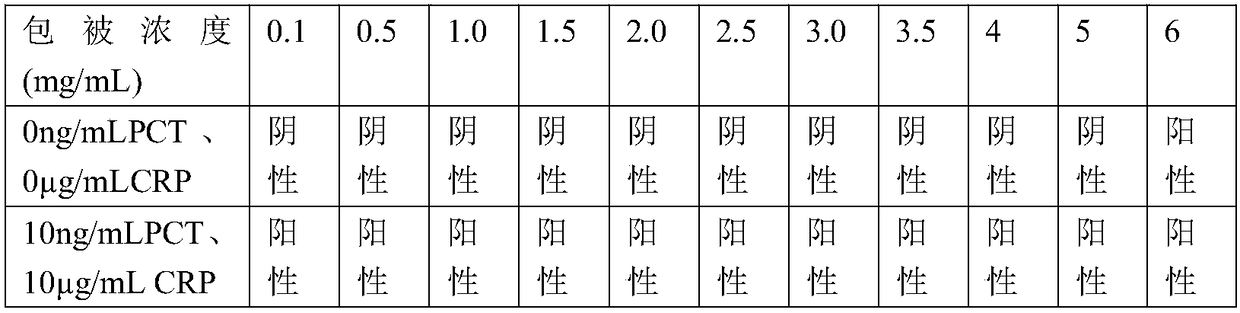
InactiveCN108845143AEasy to detectWide detection rangeBiological material analysisBiological testingFiltrationSample dilution
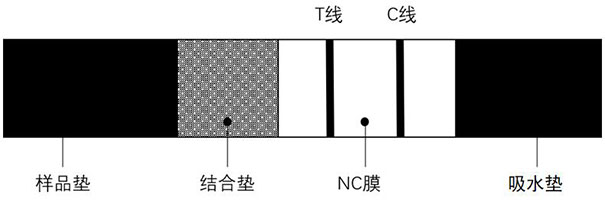
The invention provides a PCT-CRP dual card. The PCT-CRP dual card comprises a test strip substrate and a chromatographic membrane lapped on the test strip substrate, wherein a first detection line, asecond detection line and a quality control line are sequentially arranged on the chromatographic membrane. On one hand, the test strip detects PCT by a double-antibody sandwich method, and on the other hand, the test strip detects CRP by a competition method; sample dilution is not required; through simultaneous adjustment of the positions of the PCT detection line and the CRP detection line in adetection zone, interference of non-specific adsorption can be reduced; through an anti-human erythrocyte antibody in a sample pad, filtration of whole blood is achieved, and detection of a whole blood sample is achieved; in summary, the PCT-CRP dual card provided by the invention is convenient in detection, has a wider detection range, and is fast and sensitive.
Owner:ZYBIO INC
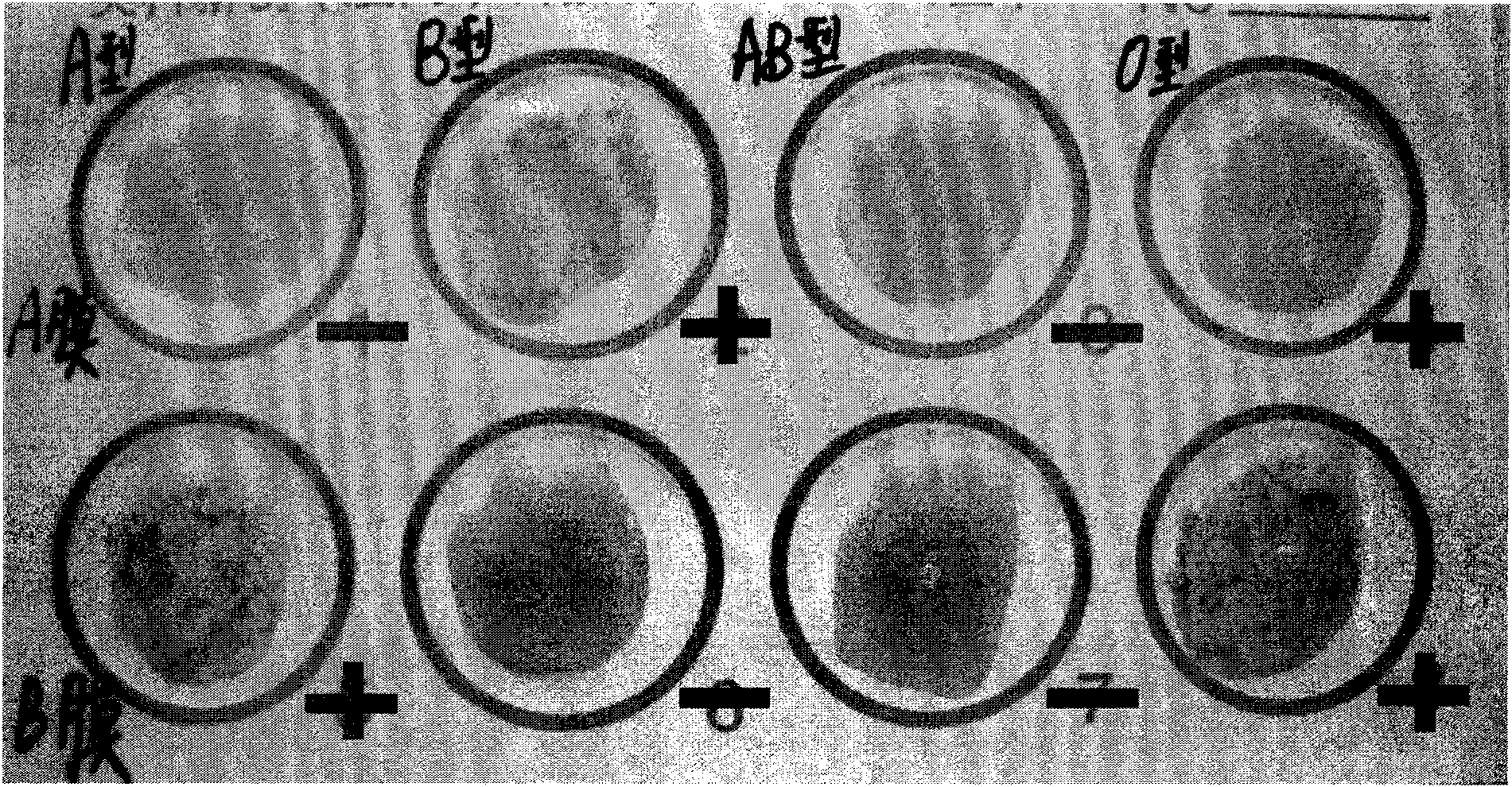
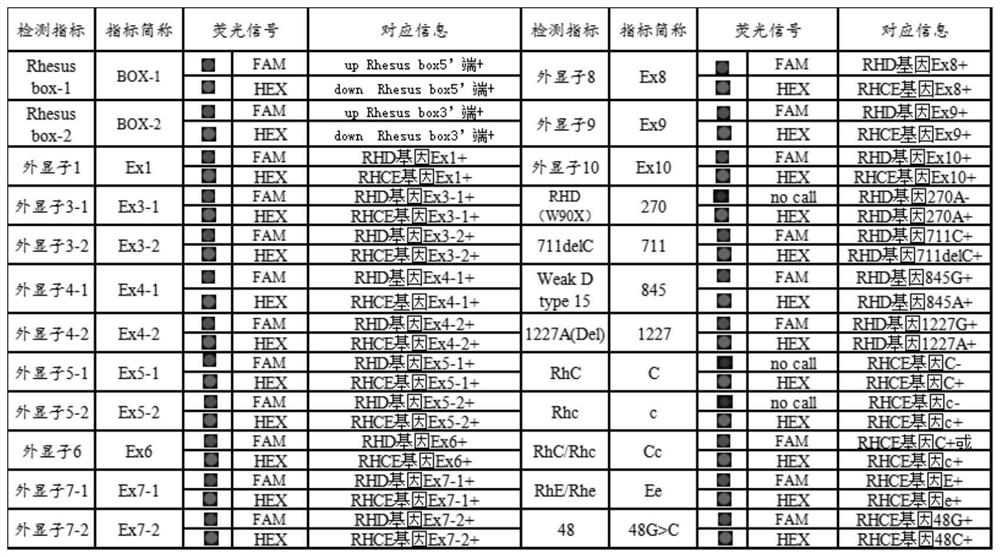
Multi-PCR-SBT genotyping method and reagent for ABO antigen of human erythrocyte blood type system
ActiveCN109554448ASimplify the experimental operation processImprove work efficiencyMicrobiological testing/measurementDNA/RNA fragmentationAntigenRed blood cell
The invention belongs to the technical field of genotyping detection method, and in particular, relates to a multi-PCR-SBT genotyping method for an ABO antigen of a human erythrocyte blood type system. The invention also relates to a multi-PCR-SBT reagent for ABO antigen genotyping of the human erythrocyte blood type system. The reagent and method provided by the invention can be used as an independent and widely used identification method, solve the problem of rapid and accurate typing of an ABO antigen system, and exert the characteristics of high-throughput operation and accurate results ofmulti-PCR-SBT on ABO genotyping. Relevant applications in the fields of clinical blood transfusion medicine research and genetics can be highly valued, and important practical significance are achieved in research departments, and pharmaceutical research and reagent development departments.
Owner:浙江省血液中心
Human red blood cell rare blood type genetic typing primer group and application
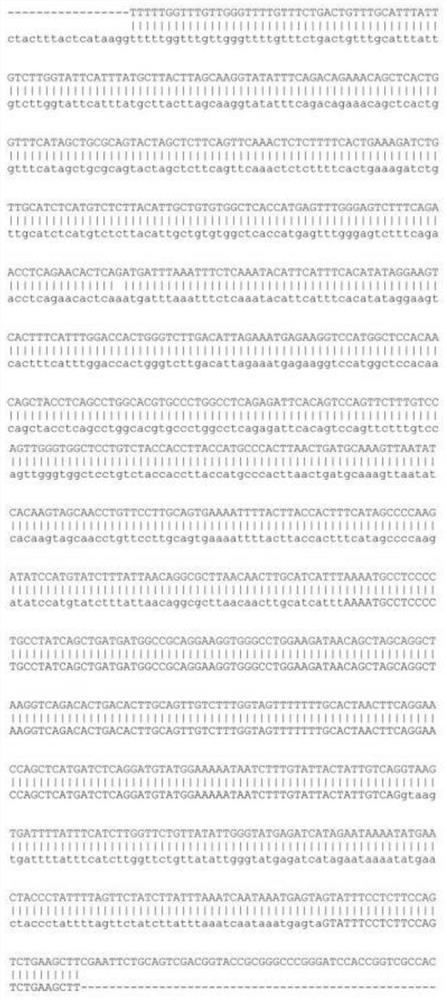
The invention discloses a human red blood cell rare blood type genetic typing primer group. The human red blood cell rare blood type genetic typing primer group comprises 43 pairs of primers as shownin SEQ ID NO. 1-86. Specific primers are designed for 12 clinically important red blood cell antigen genes, in combination with SYBR Green I, real-time fluorescent PCR amplification and melting curvereaction are carried out, when a primer sequence and a to-be-detected sequence complete each other, a target fragment is copied and amplified through PCR reaction, and then the temperature (TM) of a specific product when double strands are dissociated by 50% is detected. An internal reference (IC) is added in a PCR system, and is used for evaluating sample quality and PCR inhibiting factors.
Owner:江苏中济万泰生物医药有限公司
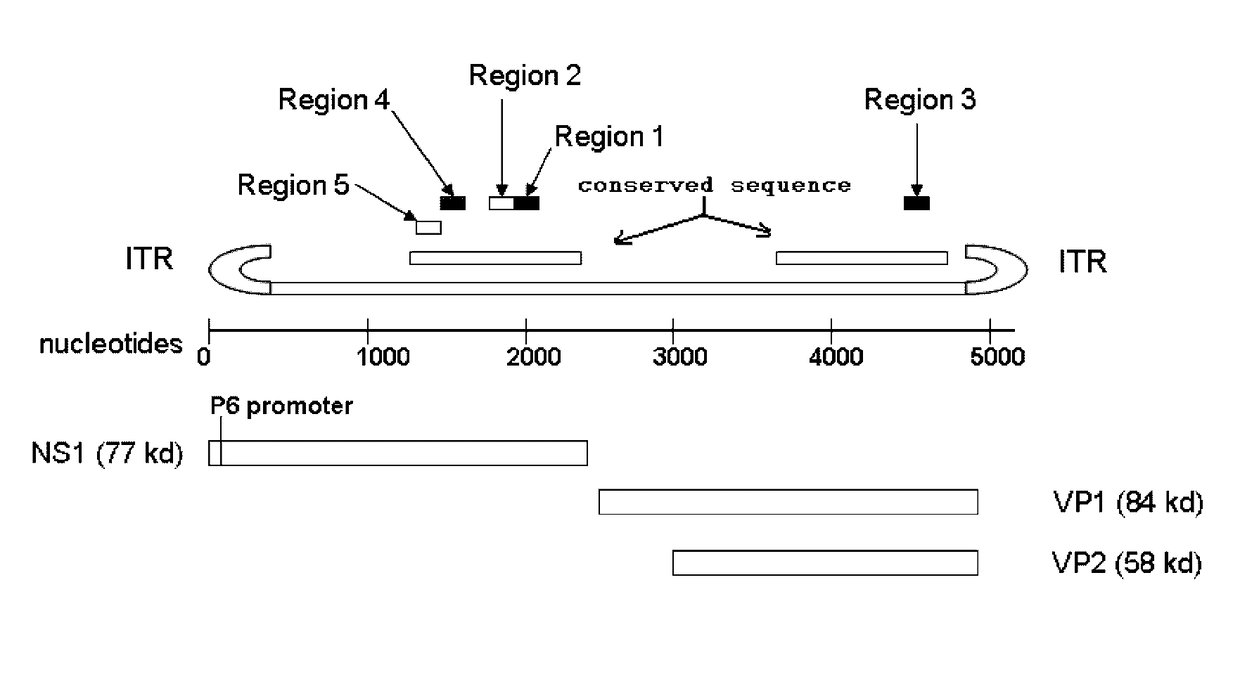
Promoter for driving specific expression of gene in human erythrocyte system and application
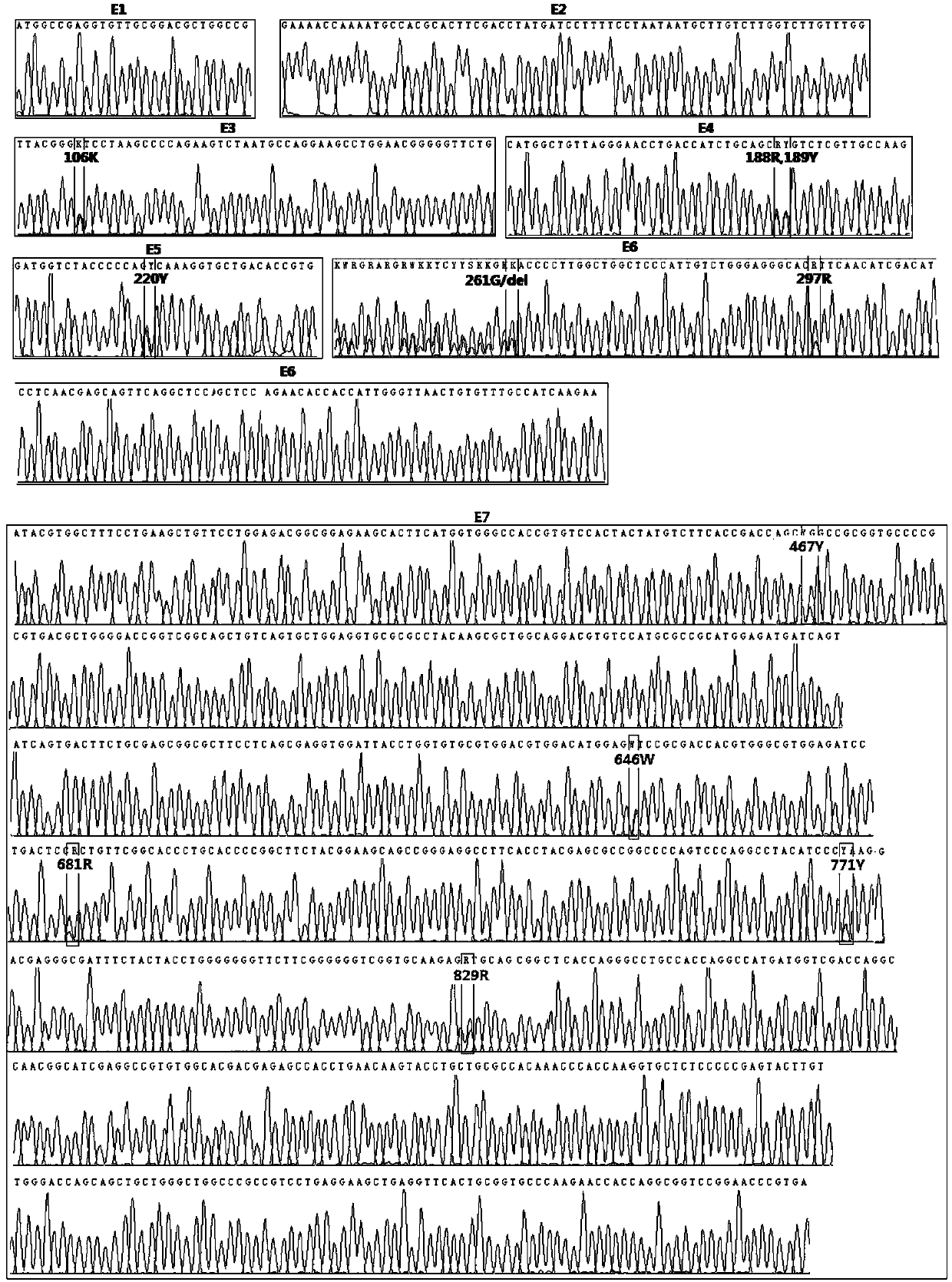
The invention provides a promoter for driving specific expression of a gene in a human erythrocyte system and application, and relates to the technical field of gene engineering. The promoter has a nucleotide sequence as shown in SEQ ID NO. 1. The promoter has functions of specifically interfering expression of a certain gene in erythroid and researching specific expression of the certain gene inthe erythroid. EGFP expression plasmids driven by promoters of different lengths are constructed by gene recombination technology, after the plasmids are introduced into HEL and Ramos cells, fluorescence expression is observed, and results show that there is no difference in activities of 1001 and 1003 in HEL cells, but the activity of 1003 is significantly higher than that of 1001 in Ramos cells,confirming that GYPA-416bp (1001) has erythroid specificity. The promoter provided by the invention has important clinical significance in directional gene therapy of erythroid system diseases and research of erythroid genes.
Owner:GUIZHOU MEDICAL UNIV
Kit for detecting folic acid content of human erythrocytes, detection method and application
ActiveCN113325171AAvoid complex operationsAvoid disadvantages that require expensive instrumentationMaterial analysisAntioxidantActive agent
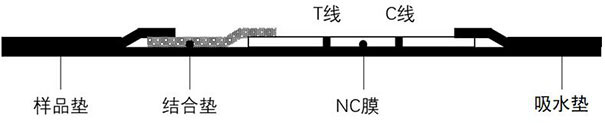
The invention relates to a kit for detecting folic acid content of human erythrocytes, a detection method and application. The invention provides a kit for detecting folic acid content of human erythrocytes. The kit comprises a whole blood sample treatment reagent, a precipitation solution and an immunochromatography test paper card, wherein the whole blood sample treatment reagent comprises an antioxidant, a surfactant and a reaction buffer solution, the immunochromatography test paper card comprises a supporting plate, a sample pad, a combination pad, a nitrocellulose membrane detection area and a water absorption pad are sequentially pasted on the supporting plate, and a marked folic acid monoclonal antibody is adsorbed on the combination pad. By adopting an immunochromatography technology, screening antibodies, optimizing sample treatment reagents and the like, the provided kit can realize the effect of simply and quickly detecting the folic acid content of the red blood cells at low cost, the defects of complicated operation or expensive instruments of the existing means are effectively overcome, and the blank of measuring the folic acid content of the red blood cells by using the immunochromatography method is filled.
Owner:天津康博尔生物基因技术有限公司
Primer groups and kit for detecting ABO genotypes of human red blood cells, and application of kit
ActiveCN110982895AReduce design difficultiesGood effectMicrobiological testing/measurementDNA/RNA fragmentationA-DNAHuman erythrocytes
The invention discloses primer groups and a kit for detecting ABO genotypes of human red blood cells, and application of the kit in reagents. 24 primer groups are disclosed in the invention, and the sequence table of the 24 primer groups is as shown in SEQ ID No. 1-72. The kit comprises at least 22 primer groups selected from the 24 primer groups. The reagents comprise the 24 group primer groups or the kit. The primers are optimally designed and have similar annealing temperatures, so the 24 groups of primers can be simultaneously amplified under the same PCR amplification condition; the volume of each PCR reaction system is only 1.15 mu L, and only 5-10 ng of a DNA template is needed, so detection efficiency and reaction sensitivity are greatly improved; and the primer groups have the advantages of high speed, low cost, high sensitivity, easy realization of multi-site joint detection and the like when used for detection.
Owner:河南兰德施坦纳基因科技有限公司
Blood purification therapeutic apparatus for treating mother-fetus Rh blood group incompatibility
ActiveCN106267407AImprove uniformityIncrease surface areaOther blood circulation devicesHaemofiltrationDiseaseHigh concentration
The invention relates to a blood purification therapeutic apparatus for treating mother-fetus Rh blood group incompatibility in the medical field. The blood purification therapeutic apparatus is characterized in that PB lysates with osmotic concentrations of 25 mmol / L and 35 mmol / L respectively are prepared; rhesus red blood cells containing a common Rh antigen of human red blood cells are alternately washed to prepare ghost cells which dialyze hemoglobin but retain the Rh antigen and can effectively adsorb an Rh antibody; purifying agents with the ghost cell concentration of 95% are prepared by using agarose, with the concentrations of 0.9%, 1.0%, 1.1%, 1.2% and 1.3% respectively, molten at 100 DEG C and then thermally insulated at 42 DEG C; from high concentration to low concentration of the agarose, 55-65ml of the purifying agents are sequentially added into a cylindrical container made of a highly-biocompatible material so as to form an adsorber in which ghost cells in the purifying agents are uniformly distributed from the sample inlet end to the sample outlet end, but agarose concentration gradually increases from the sample inlet end to the sample outlet end; the adsorber is used for clearing away the Rh antibody in plasma of a filtering purifier and macromolecular pathogenic substances generated by destroyed red blood cells so as to treat a hemolytic disease caused by the Rh blood group incompatibility.
Owner:ATTACHED OBSTETRICS & GYNECOLOGY OSPITAL MEDICALCOLLEGE ZHEJIANG UNIV +1
Detection method for human erythrocyte rare blood type genotype and detection kit
InactiveCN110079590AAccurate Blood Type ResultsComprehensive blood group resultsMicrobiological testing/measurementRed blood cellGenotype
The invention provides a detection method for human erythrocyte rare blood type genotype and a detection kit. The detection method comprises the following steps: designing a specific primer and then utilizing single-tube multiple PCR amplified reaction, thereby acquiring a target segment; designing a single-base extension primer and then performing single-base extension; adopting mass spectrometryfor accurately measuring a target DNA sequence. The gene detection method is capable of simplifying a blood compatibility test and identifying rare blood type and is an alternative of a serotype identification method. The new method is capable of helping doctors to acquire a more accurate and comprehensive blood type result and helping patients to save cost.
Owner:为康(苏州)基因科技有限公司
Industrialized production method for high galactosylated modification recombination human erythrocyte growth stimulation protein
The invention discloses an industrialized production method for high galactosylated modification recombination human erythrocyte growth stimulation protein utilizing serum-free medium used for steadily improving protein expression index of Darbepoetin Alpha. The method comprises the following steps: adopting serum-free medium to cultivate CHO cell strain in a suspending manner in a biological reactor; expressing the Darbepoetin Alpha in high efficiency in the CHO cell. The invention further discloses the serum-free medium adopted by the industrialized production method for high galactosylated modification recombination human erythrocyte growth stimulation protein. The serum-free medium component used for steadily improving protein expression index of Darbepoetin Alpha is utilized; meanwhile, the CHO cell can efficiently and steadily express Darbepoetin Alpha; expression is stable; cultivation method is brief, and is suitable for large scale cultivation.
Owner:山东丰金生物医药有限公司
Recombinant protein containing a C-terminal fragment of Plasmodium MSP-1
The invention relates to a recombinant protein fabricated in a baculovirus system, of which the essential constitutive polypeptide sequence is that of a C-terminal fragment of 19 kilodalton (p19) of the surface protein 1 (protein MSP-1) of the merozoite parasite of the Plasmodium type, particularly Plasmodium falciparum, which is infectious for humans, said C-terminal fragment remaining normally anchored at the surface of the parasite at the end of its penetration phase into human erythrocytes, in the occurrence of an infectious cycle. Said recombinant protein is applicable to the production of vaccines against malaria.
Owner:INST PASTEUR +1
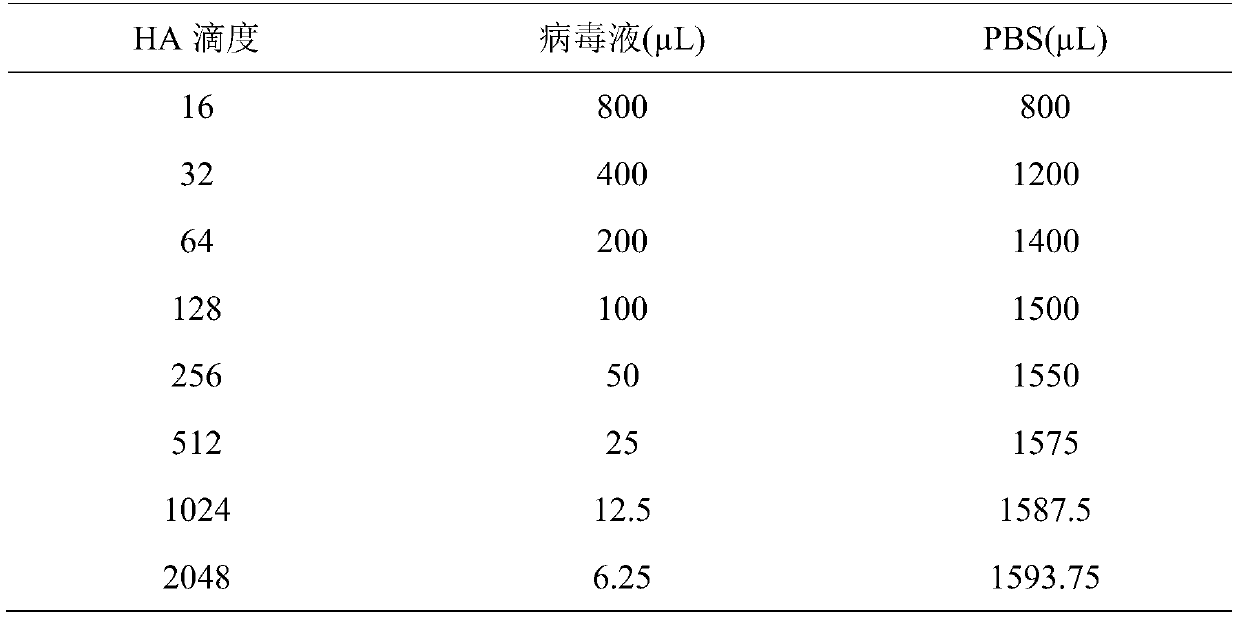
Influenza hemagglutination inhibition test detection method
PendingCN111323581ASolve the difficulty that the experiment cannot be carried outAvoid difficultiesBlood/immune system cellsBiological testingAntigenic analysisSpecific lectin
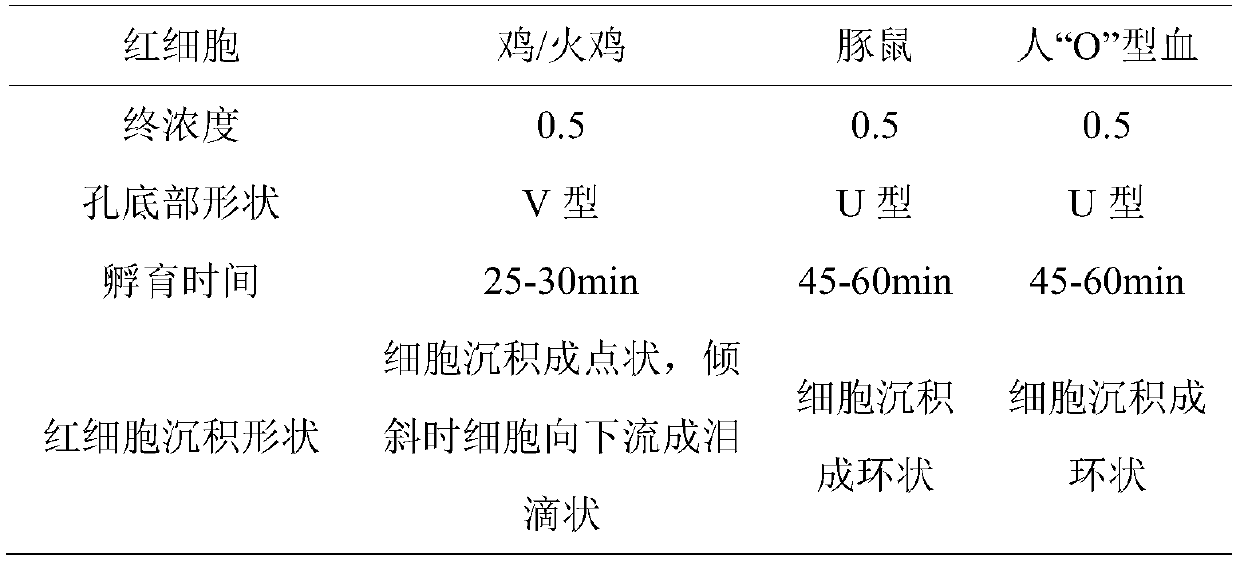
The invention belongs to the technical field of microbiological detection, and discloses an influenza hemagglutination inhibition test detection method which comprises the following steps: pretreatingstandard reference antiserum to remove non-specific inhibin and non-specific lectin in the serum; preparing an erythrocyte suspension; determining the hemagglutination titer of the influenza virus strain; preparing four hemagglutination unit antigens for an erythrocyte agglutination inhibition test; rechecking and titrating four hemagglutination unit antigens; and performing influenza virus identification and antigen analysis by using an HAI method to obtain the serum titer of the detected serum. According to the detection method, chicken erythrocyte, turkey erythrocyte and guinea pig erythrocyte are used as raw materials to prepare erythrocyte suspension, animal erythrocyte is used for replacing human erythrocyte, and the difficulty that an experiment cannot be conducted due to lack of erythrocyte is solved.
Owner:广州鸿泉生物科技有限公司
Injection for treating cancer and its preparation method
InactiveCN101327317BGrowth inhibitionPeptide/protein ingredientsPharmaceutical delivery mechanismErythrocyte membraneRed blood cell
The invention relates to the use of human erythrocyte membrane during the preparation of the medicine for remedying tumors, also provides injection which embodies the use. The injection comprises human erythrocyte membrane albumen and buffer solution. The concentration of the human erythrocyte membrane albumen in the buffer solution is 10mg / ml-30mg / ml. The buffer solution is PBS or physiological saline solution. The injection of the invention can cure various solid tumors.
Owner:SOUTHERN MEDICAL UNIVERSITY
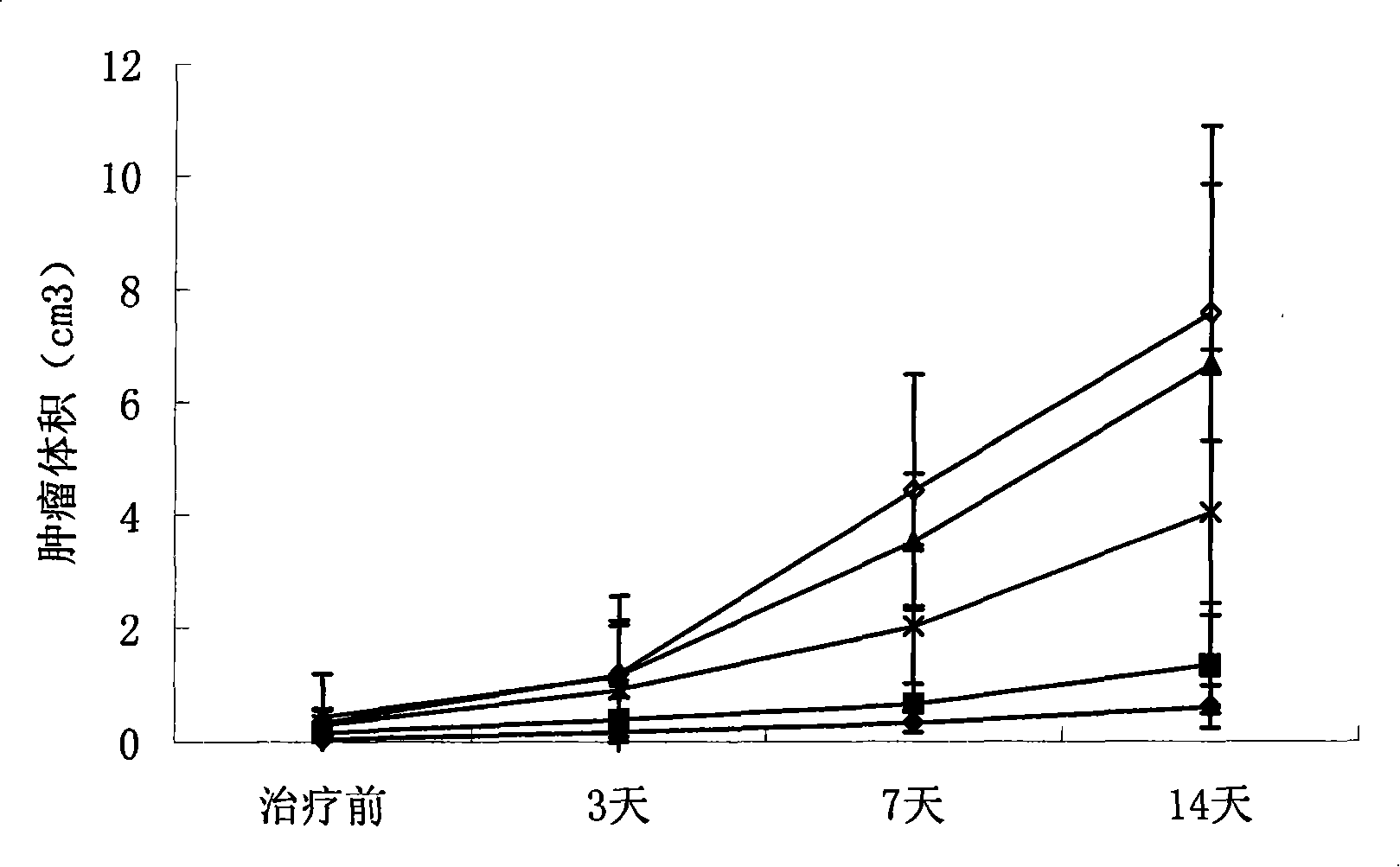
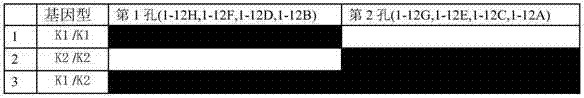
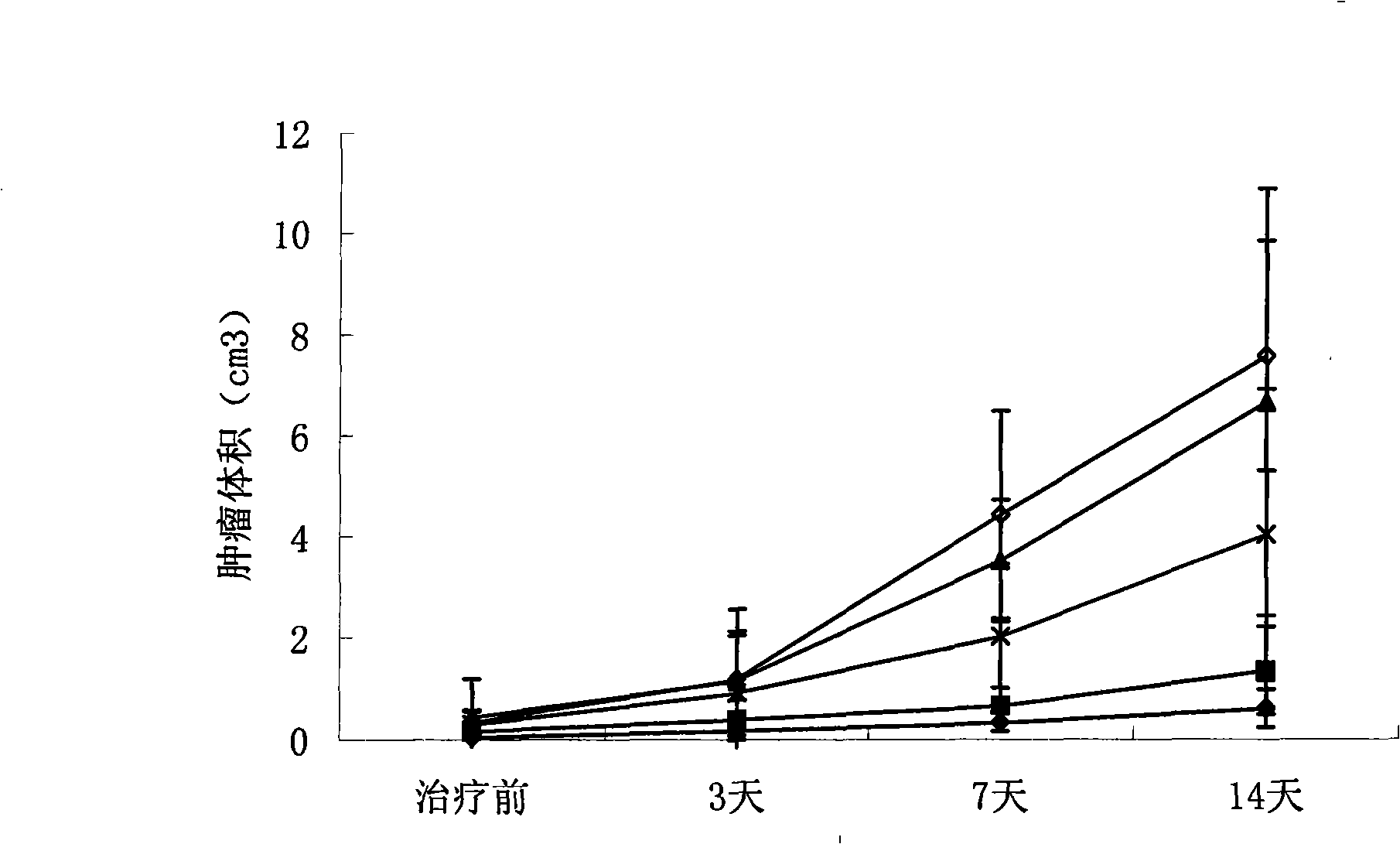
Primer group and kit for detecting human erythrocyte Kell blood type genotyping
InactiveCN104726586AExpensive to fixOvercome the shortcomings such as bad purchasesMicrobiological testing/measurementDNA/RNA fragmentationGroup A - bloodGenotyping
The invention discloses a primer group and a kit for detecting human erythrocyte Kell blood type genotyping. The primer group for determining a Kell blood type gene comprises a K1 primer pair, a K2 primer pair and an internal reference primer pair. According to the kit disclosed by the invention, restrictions in authentication of Kell blood type by a serum method are overcome; and the kit is indispensable auxiliary means for correct judgment, and has wide application prospect and clinical reference value.
Owner:天津市秀鹏生物技术开发有限公司
Use of human erythrocyte membrane protein of foreign body in preparing medicament for treating cancer
InactiveCN101327317AImprove toleranceSignificant antitumor immunityPeptide/protein ingredientsPharmaceutical delivery mechanismErythrocyte membraneRed blood cell
The invention relates to the use of human erythrocyte membrane during the preparation of the medicine for remedying tumors, also provides injection which embodies the use. The injection comprises human erythrocyte membrane albumen and buffer solution. The concentration of the human erythrocyte membrane albumen in the buffer solution is 10mg / ml-30mg / ml. The buffer solution is PBS or physiological saline solution. The injection of the invention can cure various solid tumors.
Owner:SOUTHERN MEDICAL UNIVERSITY
Erythrocyte cryopreservation method and cryopreserved erythrocytes
ActiveCN112616827AHigh recovery rateGood freezing effectDead animal preservationBlood/immune system cellsBiotechnologyCryopreservation
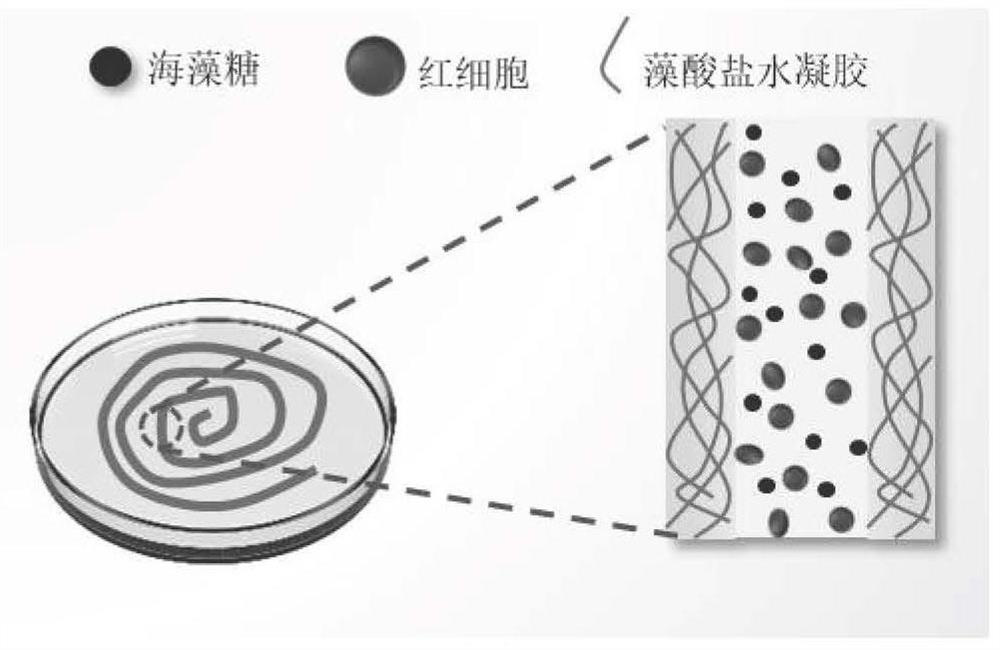
The invention relates to the technical field of erythrocyte preservation, in particular to an erythrocyte cryopreservation method and cryopreserved erythrocytes. An erythrocyte low-temperature protective agent used in the invention is an impermeable protective agent, and under the protection of the impermeable protective agent, erythrocytes are wrapped in a gel shell structure formed by cross-linking of sodium alginate to form fibers containing erythrocytes. Compared with the existing erythrocyte cryopreservation technology, the erythrocyte cryopreservation scheme provided by the invention has the advantages that the tedious glycerol adding and deglyceridation processes are omitted, the survival rate of the frozen human erythrocytes in glycerol-free quick cryopreservation can reach 90% or above, and meanwhile, the erythrocytes can keep a good microscopic form.
Owner:UNIV OF SCI & TECH OF CHINA
Storage-stable cellular whole blood composition containing elevated amounts of D-dimer
ActiveUS7560283B2Mammal material medical ingredientsArtificial cell constructsRed blood cellBlood plasma
The present invention provides a cellular whole-blood D-dimer composition for use with diagnostic test procedures for D-dimer, and a method of its preparation. The composition comprises human erythrocytes and processed plasma to which D-dimer, and stabilizers, and optionally antimicrobial agents, are added and can be used as a standard and control for D-dimer testing.
Owner:BIO RAD LAB INC
Nutrient medium for cultivating bacteria
The invention relates to microbiology, and more particularly to nutrient media used for cultivating bacteria for the subsequent study thereof. A nutrient medium comprising a pancreatic digest of casein, a peptic digest of meat, a heart pancreatic digest, yeast extract, starch and water is characterized in that it additionally contains violuric acid and beef infusion, wherein the ratio of ingredients is (wt %):0.3-1.0 pancreatic digest of casein; 0.1-1.5 peptic digest of meat; 0.1-0.9 heart pancreatic digest; 0.1-2.0 yeast extract, 0.3-0.8 starch; 0.001-0.05 violuric acid; 2.0-15 beef infusion; the remainder water. The nutrient medium can additionally contain 0.3-2.5 wt % agar-agar and / or 1-20 wt % whole or hemolyzed sheep red blood cells and / or 1-20 wt % whole or hemolyzed human red blood cells and / or 1-15 wt % horse blood serum. This provides for the simultaneous growth of the maximum possible number of bacteria present in an inoculate.
Owner:VIKTOR VENIAMINOVICH TETAB +1
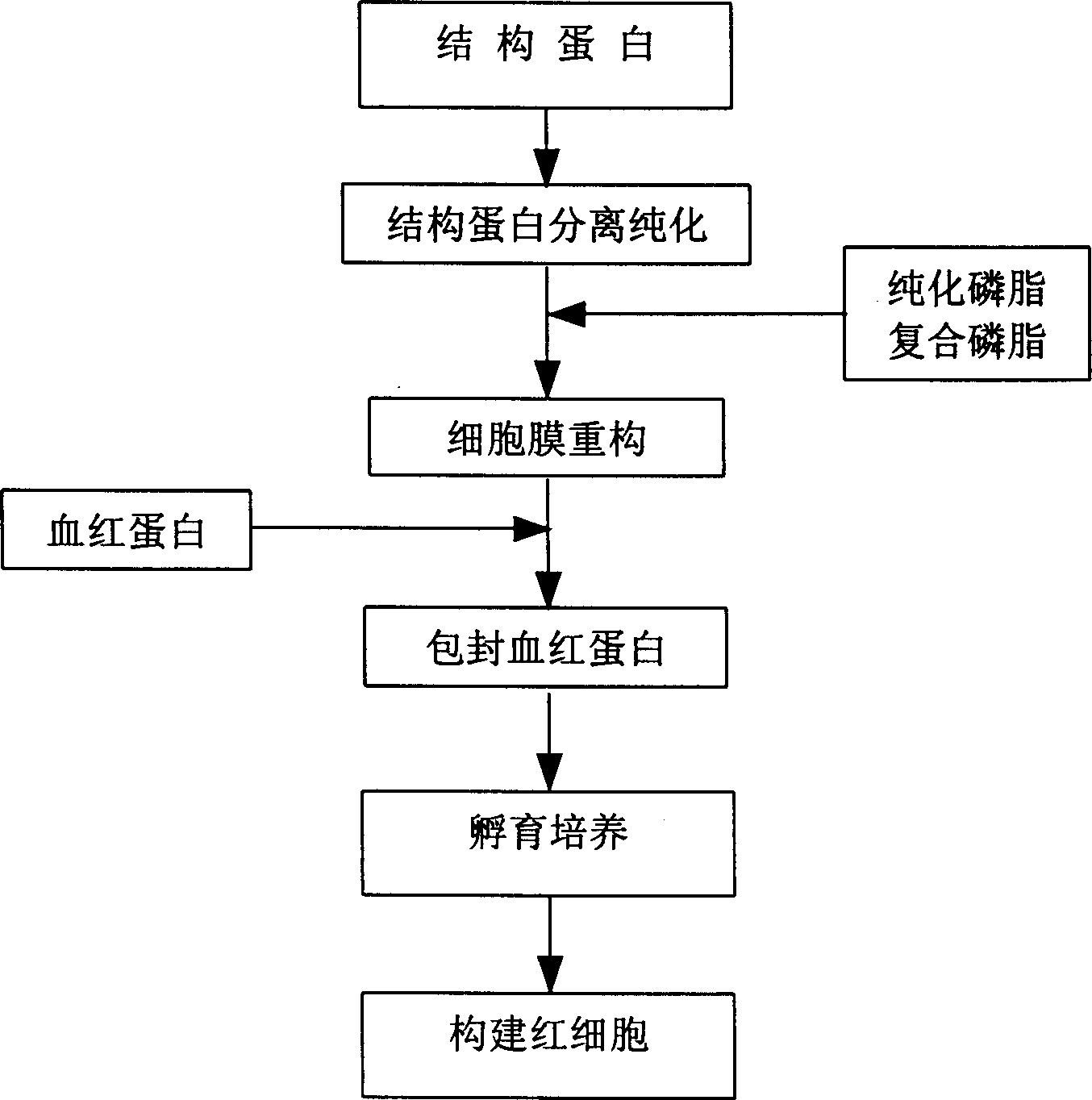
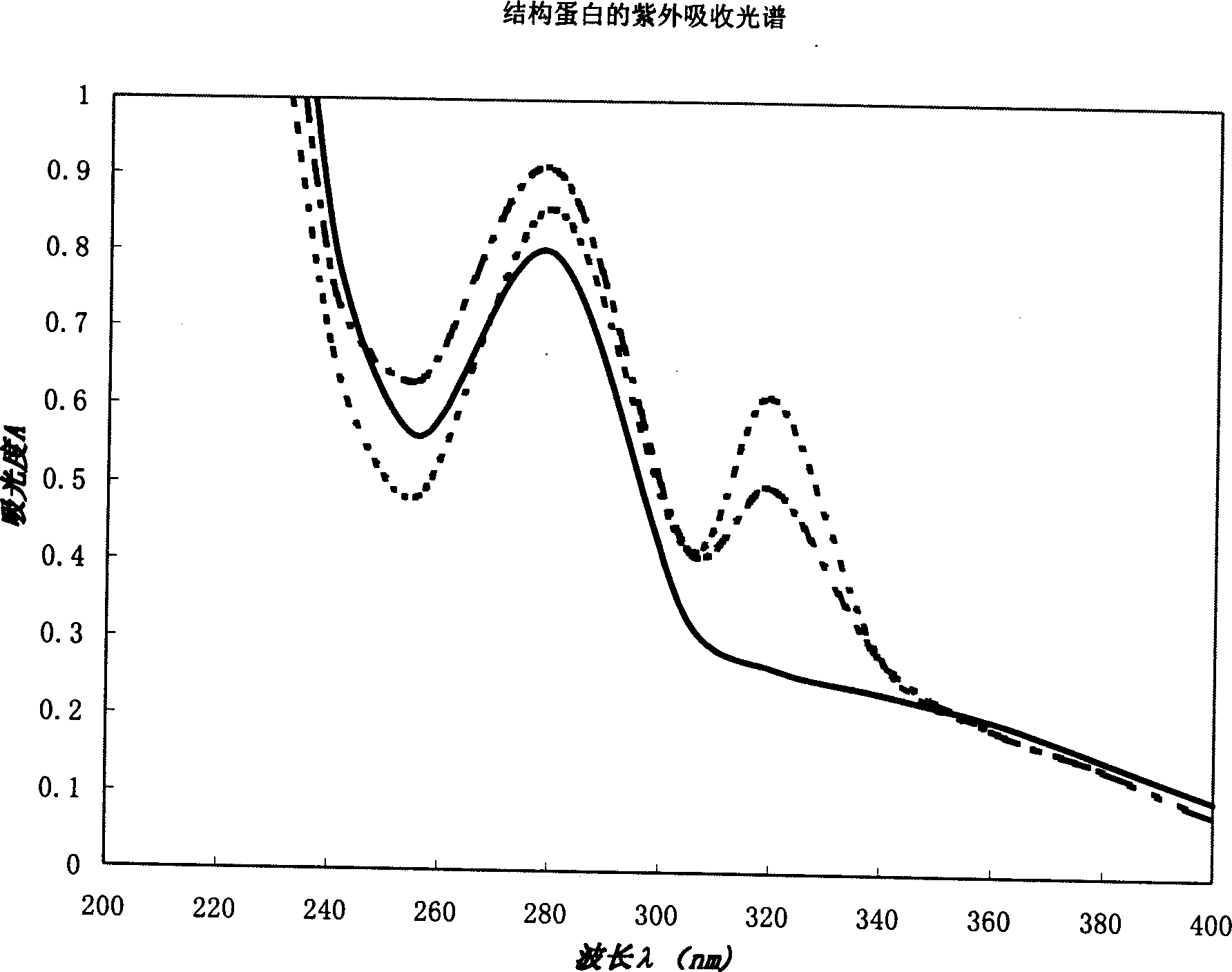
In vitro reconstituted human erythrocyte, its preparation and application in blood substitute material
A human red cell recombined in vitro, its preparing process and it application to blood substitute are disclosed. The said human red cell is composed of haematoglobin, structural protein and phospholipid and has the physiological function and form, which are similar to that of natural red cell. Its advantages are good deformability, good stability, strong oxygen carrying and releasing power, and controllable size.
Owner:FEIXIANG BIOTECH CHONGQING
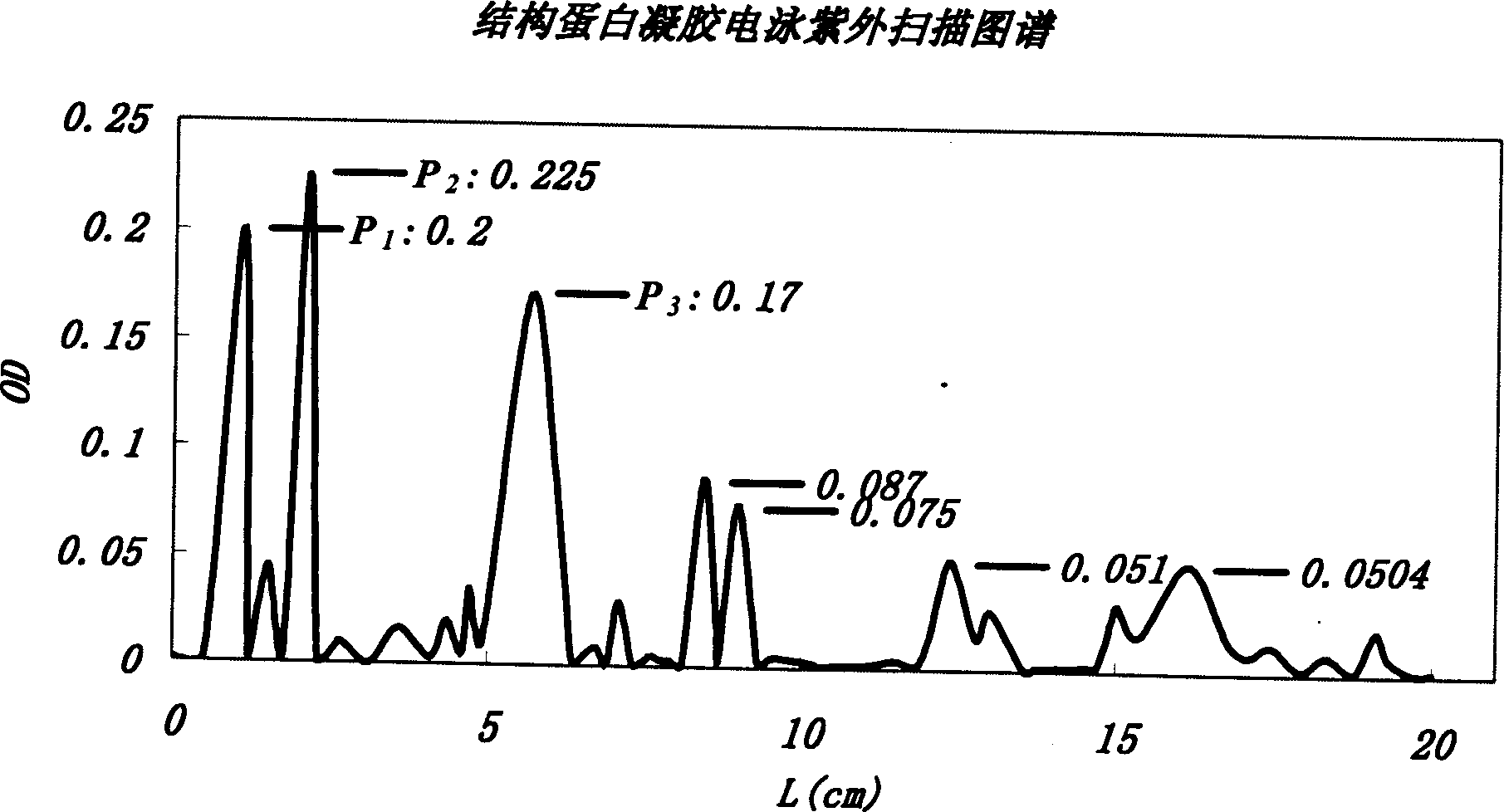
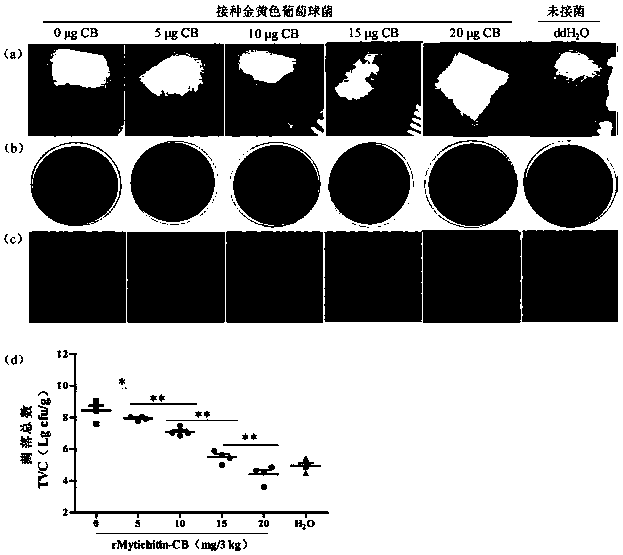
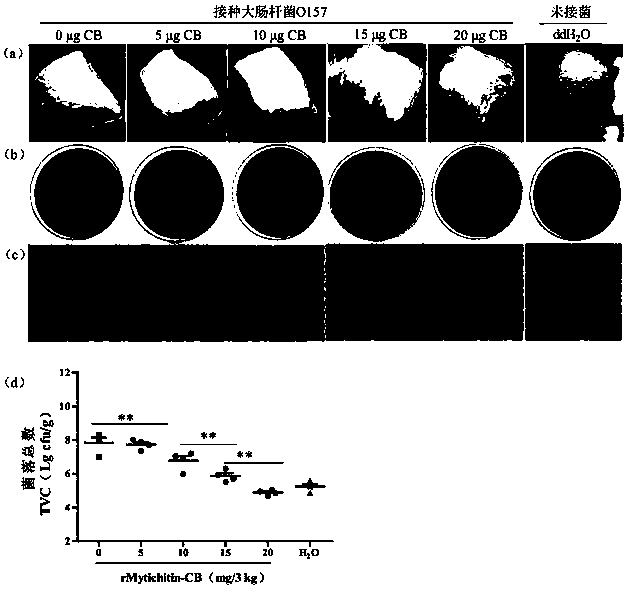
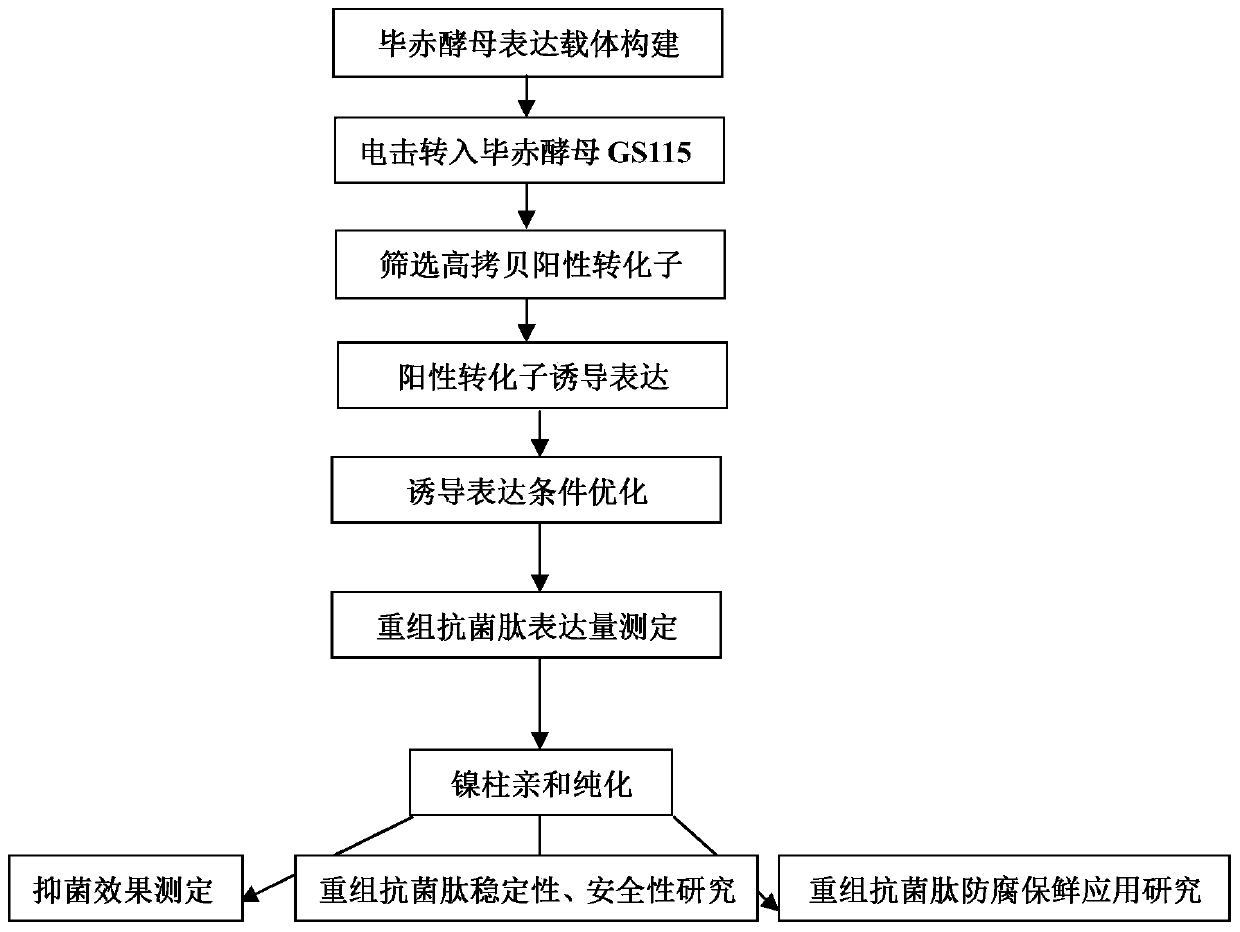
Application of recombinant Mytichitin-CB antimicrobial peptide
InactiveCN110156884AGood anti-corrosion and fresh-keeping effectGood sensory formMicroorganism based processesPeptide preparation methodsBiotechnologyRed blood cell
The invention belongs to the field of biotechnology, and particularly relates to application of recombinant Mytichitin-CB antimicrobial peptide (rMytichitin-CB). The amino acid sequence of the antimicrobial peptide is as shown in the sequence table SEQ ID NO:1, and has good temperature stability, pH stability and relative protease stability; the hemolytic activity of human erythrocyte, mouse erythrocyte and chicken erythrocyte is small, the possibility of application in the fields of food, animal husbandry, medicine and the like is achieved; the antimicrobial peptide can be widely applied to preservation and fresh keeping of fresh food, reproduction of microorganisms is inhibited, and the loss of nutrients is lowered.
Owner:TIANJIN UNIV OF SCI & TECH
Human erythrovirus
Nucleic acid molecules derived from sequences of novel human parvovirus B19 variant genomes are provided. Also provided are assays and kits comprising the nucleic acid molecules.
Owner:GRIFOLS THERAPEUTICS LLC
Primer combination for detecting Diego blood group genotyping of human red cells and kit
InactiveCN104694656AExpensive to fixOvercome the shortcomings such as bad purchasesMicrobiological testing/measurementDNA/RNA fragmentationGroup A - bloodGenotyping
The invention discloses a primer combination for detecting the Diego blood group genotyping of human red cells and a kit. The primer combination for detecting Diego blood group genotyping of human red cells comprises a Dia primer pair, a Dib primer pair and an internal primer pair. According to the kit provided by the invention, various limitations on Diego blood type determined by a serologic method can be overcome, therefore the primer combination is an indispensable assistant means of accurately judging the blood type, and has an extensive application prospect and a clinical reference value.
Owner:天津市秀鹏生物技术开发有限公司
Primer group and kit for detecting human red blood cell Rh blood group genotyping and application
PendingCN114507724AReduce volumeLow costMicrobiological testing/measurementDNA/RNA fragmentationMedicineGroup A - blood
The invention discloses a primer group and a kit for detecting blood type gene typing of human red blood cells Rh and application of the primer group. A sequence table of the primer group is as shown in SEQ ID No.1 to 9, SEQ ID No.10 to 12 or / and SEQ ID No.13 to 15, SEQ ID No.16 to 18 or / and SEQ ID No.19 to 21, SEQ ID No.22 to 24 or / and SEQ ID No.25 to 27, SEQ ID No.28 to 30, SEQ ID No.31 to 33 or / and SEQ ID No.34 to 36 and SEQ ID No.37 to 72. The primers are subjected to optimization design and have similar annealing temperatures, simultaneous amplification of all the primers under the same PCR amplification condition can be achieved, the volume of each PCR reaction system is only 1.15 microliters, only 5-10 ng of a DNA template is needed, and the detection efficiency and the reaction sensitivity are greatly improved; the primer group has the advantages of high speed, low cost, high sensitivity, easiness in realizing multi-site joint detection and the like when being applied to detection.
Owner:河南兰德施坦纳基因科技有限公司
A filter blood sample pad and its preparation method
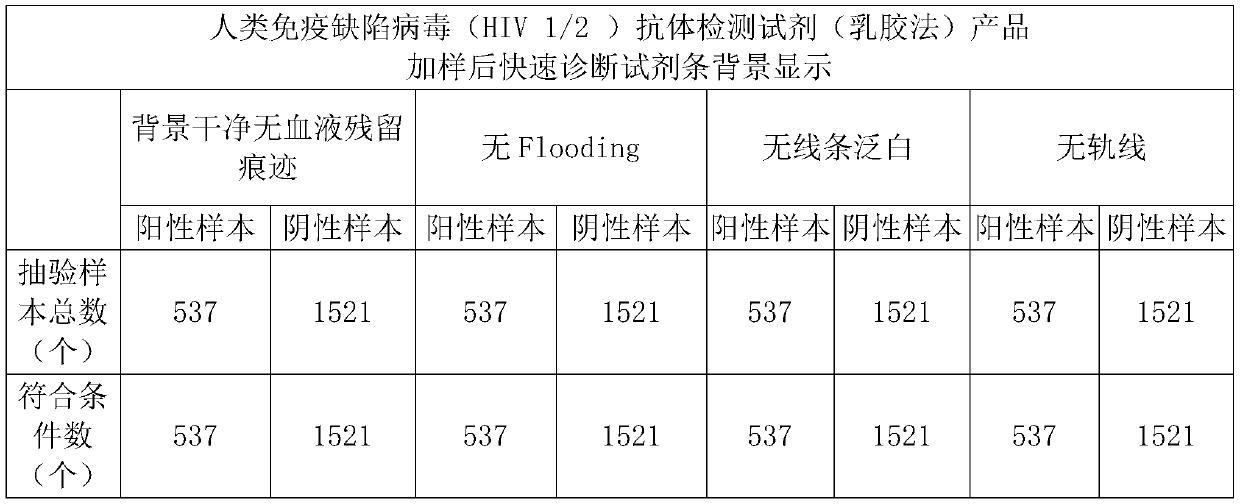
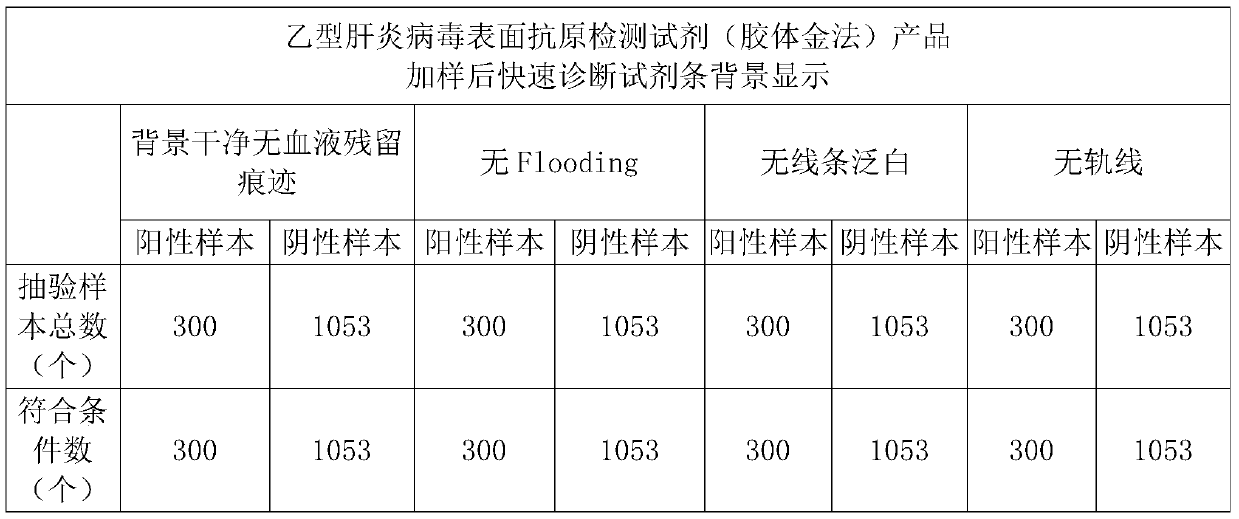
ActiveCN106442079BEasy to separateNo blood residuePreparing sample for investigationAntiendomysial antibodiesActive agent
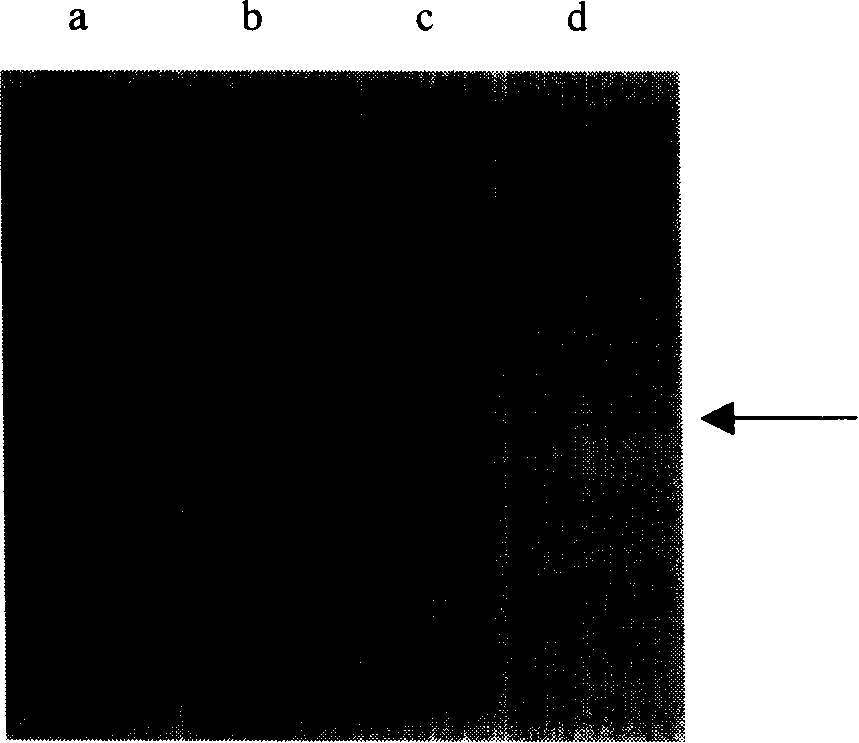
The invention relates to a hemofiltration sample pad. According to a preparation mode, a certain proportion of human erythrocyte antibodies, trihydroxymethyl aminomethane, casein, polyvinylpyrrolidone, surfactants and the like are prepared into a treatment solution according to different product properties in accordance with different sequences; the prepared solution is subjected to pH regulation so as to form a final treatment solution; the prepared treatment solution is uniformly processed on glass fiber and is put into a 37-DEG C drying box to be dried for 8 to 24 hours. The hemofiltration sample pad prepared by the method has the advantages that the whole blood sample is separated under the condition of not influencing the sample running board; a fast diagnosis reagent subjected to sample feeding has clean background without phenomena such as blood residue traces, flooding, line whitening or rail lines.
Owner:HANGZHOU BIOTEST BIOTECH CO LTD
Hybridoma cell line and anti-human erythrocyte surface H antigen monoclonal antibodies generated thereof
InactiveCN101037671BImmunoglobulins against cell receptors/antigens/surface-determinantsFused cellsErythroid cellCell strain
The invention discloses a hybridization tumor cell stem 2E8Z CGMCC NO.1942 and monoclonal antibody of human red blood cell surface H antigen thereof. The monoclonal antibody 2E8 of human red blood cell surface H antigen genereated by hybridization tumor cell stem has advantages of high effect, strong differentia, extensive distribution of antigen. The flexible peptide is easy to be bended, so mixed albumen of the invnetion is feld correctly and without influence each activity combination area of the mixed albumen. Experiment evidence, the mixed albumen can specially combine with human red blood cell surface H antigen; moreover, the mixed albumen with high expression amount, easy to be purified, short production cycle, large production scale and low cost. Base on these advantages, the invention plans to use gene engineering method to produce double function molecule and establish good base of treating based on red blood cell and testing platform, having more actual sense and wider application foreground in medical testing field.
Owner:FIELD OPERATION BLOOD TRANSFUSION INST OF PLA SCI ACAD OF MILITARY
Method for preventing or treating thrombosis
ActiveUS20220080009A1Avoid loweringPrevention or treatment/alleviation of thrombosisOrganic active ingredientsBlood disorderCannabisThrombus
Disclosed is a method for preventing or treating thrombosis, the method comprising administering a composition containing a hemp (Cannabis sativa L.) leaf or flower extract as an active ingredient. The hemp (Cannabis sativa L.) leaf or flower extract inhibits thrombus formation-related enzymes and coagulation factors and exhibits potent antithrombotic activity through inhibitory activity against platelet aggregation which triggers blood clotting, but with no lytic activity on human erythrocytes. In addition to being stable to heat, the extract does not lose the inhibitory activity against coagulation factors and thrombus formation-related enzymes even in the acidic condition of pH 2 and the plasma environment. Therefore, the extract is expected to find applications in preventing and treating thrombosis such as ischemic stroke and hemorrhagic stroke through blood circulation improvement.
Owner:ANDONG NAT UNIV IND -ACADEMIC COOP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com