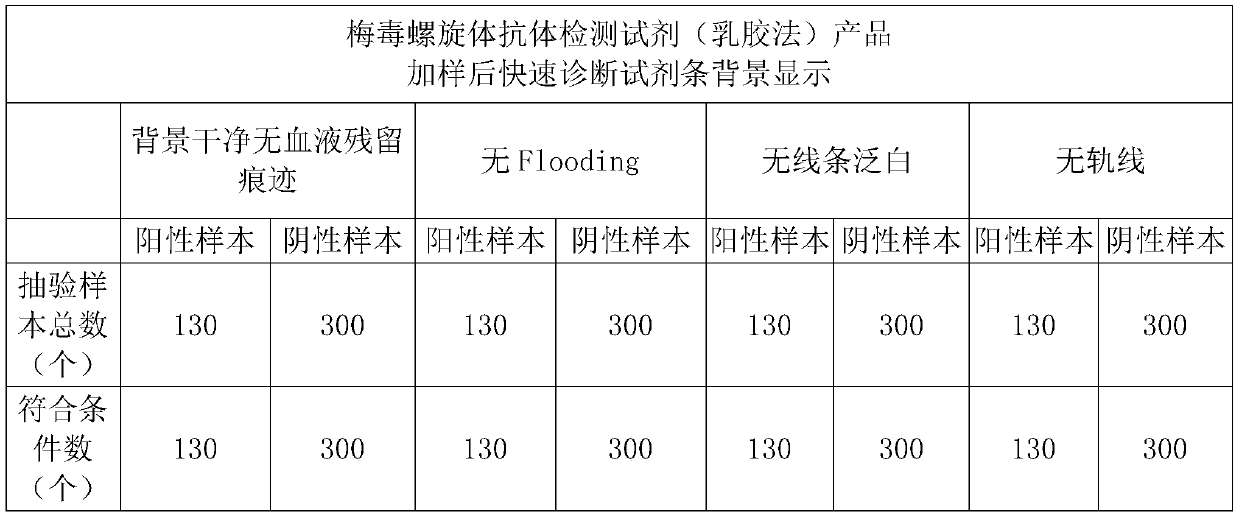
A filter blood sample pad and its preparation method
A sample pad and blood filtration technology, applied in the field of medical testing, can solve problems such as affecting the speed of testing samples and testing results, increasing the thickness of reagent strips, and unfavorable production operations, so as to improve solubility, improve uniformity, and promote enterprises. effect of development
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0036] The preparation steps of the sample pad are as follows:
[0037] 1. According to the performance of different products, the raw materials of each component are prepared into a solution according to the corresponding proportion and order, and the pH value of the prepared solution is adjusted to form the best buffer system suitable for the antigen-antibody reaction;
[0038] 2. Calculate the required amount of solution according to the corresponding glass fiber liquid absorption coefficient, and evenly treat the prepared treatment solution on the glass fiber;
[0039] 3. Dry the glass fibers treated with the treatment solution in an oven or drying room at 37°C for 8-24 hours.
Embodiment 1
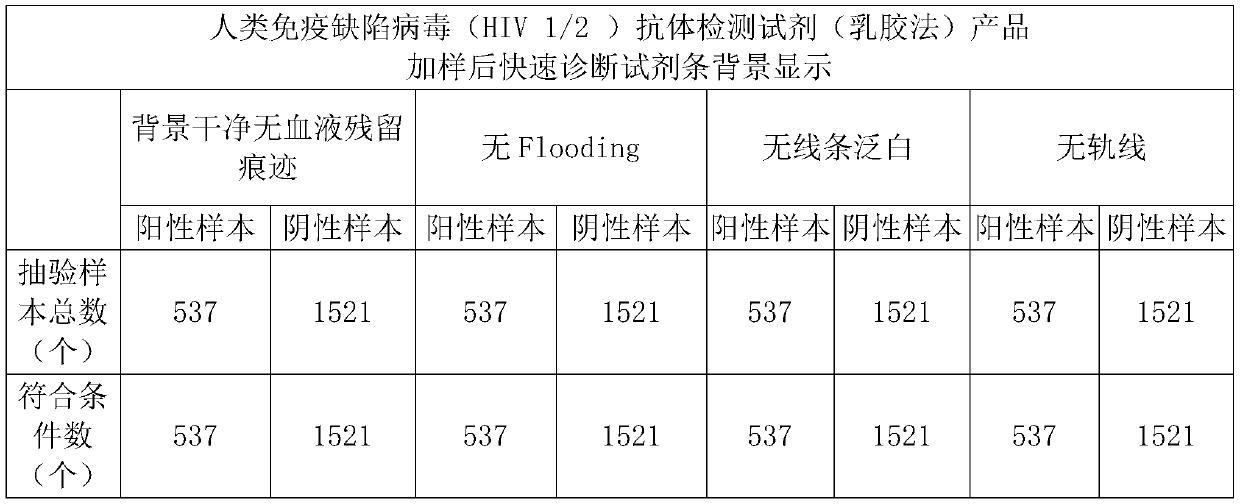
[0040] Embodiment 1: the blood filter sample pad processing of human immunodeficiency virus (HIV 1 / 2) antibody detection reagent (latex method) product
[0041] The preparation steps of the filtered blood sample pad are as follows:
[0042] 1) Prepare the required amount of 0.9 purified water for the solution, add 0.04-0.06M Tris, 0.15-0.25mM Casein, 0.15-0.35mM PVP in sequence, stir the previous component to completely dissolve and then add the next component, Stir until completely dissolved;
[0043] 2) Add in order to the solution prepared in step 1): 0.01-0.015M sodium carbonate (hereinafter abbreviated as: Na 2 CO 3 ), 0.6 to 1.1% of the required treatment solution, 3 to 5% of the mouse-derived anti-RBC in the required treatment solution, the previous component was stirred and completely dissolved, and then the next component was added, and the solution was stirred until it was completely dissolved. clarify;
[0044] 3) adjust the pH value to 8.0±0.1 with hydrochloric...
Embodiment 2
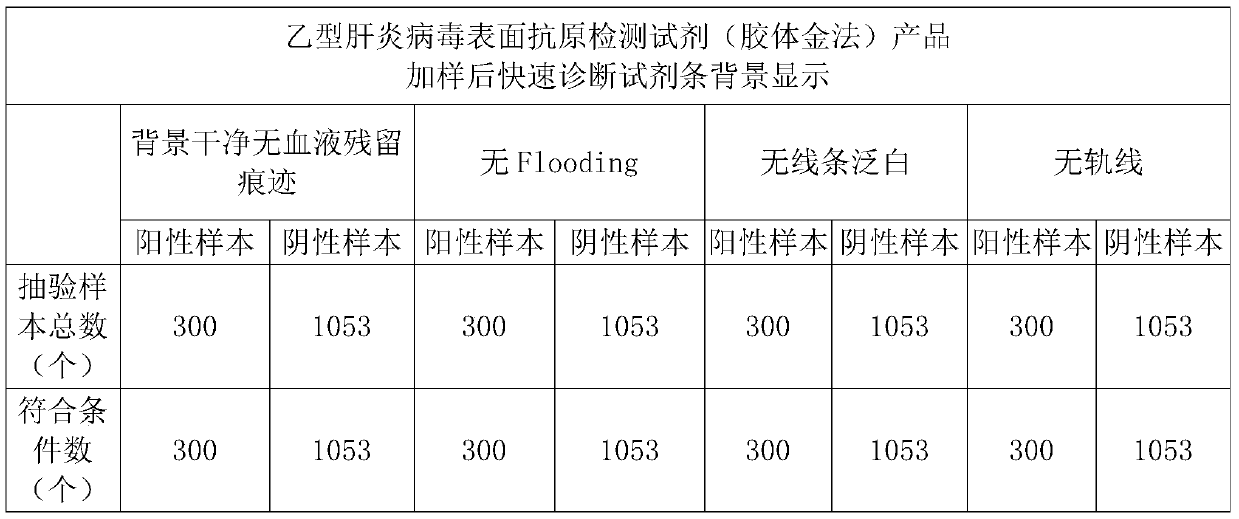
[0047] Embodiment 2: Hepatitis B virus surface antigen detection reagent (colloidal gold method) product sample pad processing
[0048] The preparation steps of the sample pad are as follows:
[0049] 1) Take 0.7% purified water required for the treatment solution, add 0.04-0.06M Tris, 0.1-0.3mM PVP, 0.02-0.1% surfactant, 0.15-0.25mM PVP to the purified water in order Casein, 0.02-0.04M ethylenediaminetetraacetic acid, 8-12% protein stabilizer in the required treatment solution, 2-5% sheep-derived anti-RBC in the required treatment solution, after the former component is stirred and completely dissolved Add the next component and stir until completely dissolved and the solution is clear;
[0050]2) Adjust the pH value of the solution to 8.0±0.1 with hydrochloric acid or sodium hydroxide solution;
[0051] 3) Dilute the solution to the required solution volume with purified water, and retest the pH value at 8.0±0.1;
[0052] 4) Evenly treat the prepared treatment solution on...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com