Industrialized production method for high galactosylated modification recombination human erythrocyte growth stimulation protein
A cell and process technology, applied in the field of biomedicine, can solve the problems of clinical drug safety hazards, increase the possibility of unknown virus contamination of products, and difficulty in ensuring product quality uniformity. The effect of improving safety and uniformity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] This embodiment provides a serum-free medium, which includes a serum-free basal medium and additives.
[0040] The serum-free basal medium ratio of the present embodiment is determined like this:
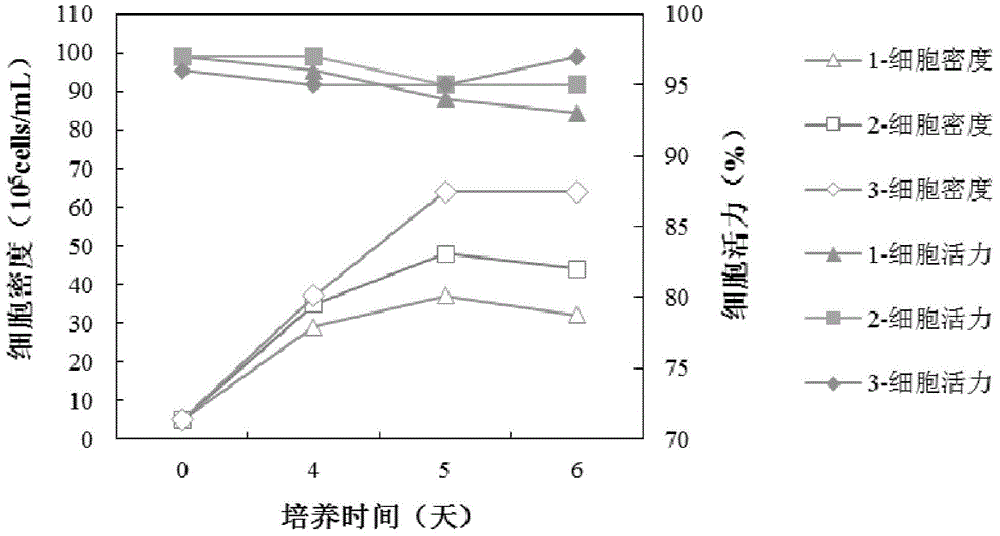
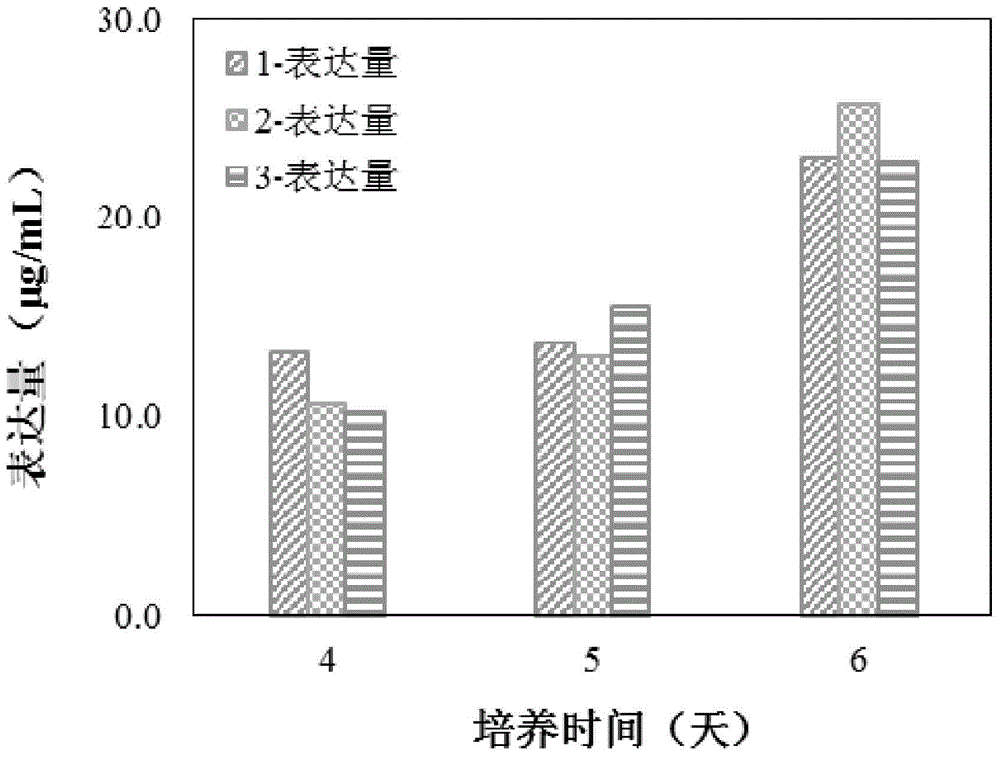
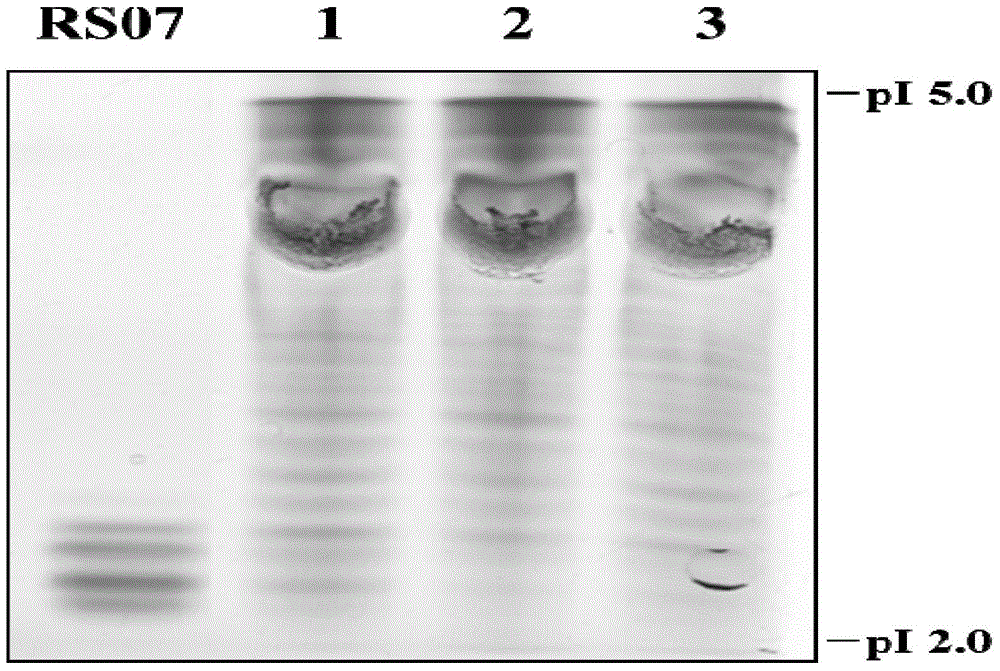
[0041] Resuscitate the seed cells and amplify the culture, prepare the cell suspension, and inoculate them in three serum-free mediums 1, 2, and 3 respectively. The weight ratios of medium 1, 2, and 3 are 9:1, 3:1, respectively. , 1:1 combination medium of Ex-cell302 medium and CP1027 medium. Cultivate at 36+0.2°C, conduct 6-day sugar batch experiment, start from the 4th day of culture, count the cells every day, collect the culture supernatant, centrifuge to remove the cells and freeze them, and use the ELISA method to detect the expression level of the cells. The proportion of the acidic part of the cell expression product was detected by the method of IEF. In the evaluation, the proportion of the acidic part is taken as the primary consideration index. Evaluation result...
Embodiment 2
[0046] This example provides an industrial production method of highly glycosylated recombinant human erythrocyte growth-stimulating protein, using the serum-free medium of Example 1, and adopting a 5L reactor of animal cells for high-density, suspension perfusion culture, including the following step:
[0047] 1. Cell inoculation stage control
[0048] Resuscitate a 20mL CHO seed cell into a shaker flask, subculture and expand every 3 days, until the cell is expanded to 500mL, and the cell is in the logarithmic growth phase (the density is about 3×106cells / mL), inoculate the 500ml seed cell To the serum-free medium in the 5L NBS CELLIGEN310 bioreactor, the stirring speed is 100-200 rpm. The cell density after inoculation was 5.1×10 5 cells / mL, the initial working volume of the reactor was 3L, and the serum-free medium inoculated with CHO cells was obtained.
[0049] 2. Cell growth stage control
[0050] Under the conditions of temperature 36+0.2°C, pH 6.90-7.20, and disso...
Embodiment 3
[0057] This example provides an industrial production method of highly glycosylated recombinant human erythrocyte growth-stimulating protein, using the serum-free medium of Example 1, adopting high-density, suspension perfusion culture of animal cells in a 30L reactor, including the following steps :
[0058] 1. Cell inoculation stage control
[0059] Resuscitate a 20mL CHO seeded cell into the shake flask, subculture and expand every 3 days, until the cell is expanded to 5000mL, and the cell is in the logarithmic growth phase (the density is 3×10 6 cells / mL), the 5000ml seed cells were inoculated into the serum-free medium in the Sartourius Stedium C-Plus30L bioreactor, and the stirring speed was 100-200 rpm. The cell density after inoculation was 6.6×10 5 cells / mL, the initial working volume of the reactor was 30L, and a serum-free medium inoculated with CHO cells was obtained.
[0060] 2. Cell growth stage control
[0061] Under the conditions of temperature 36+0.2°C, p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com