Application of recombinant Mytichitin-CB antimicrobial peptide
A technology of antimicrobial peptide and fresh-keeping solution, which is applied in the field of recombinant Mytichitin-CB antimicrobial peptide and its high-efficiency preparation, which can solve the problems of no public use and achieve good temperature stability and good inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
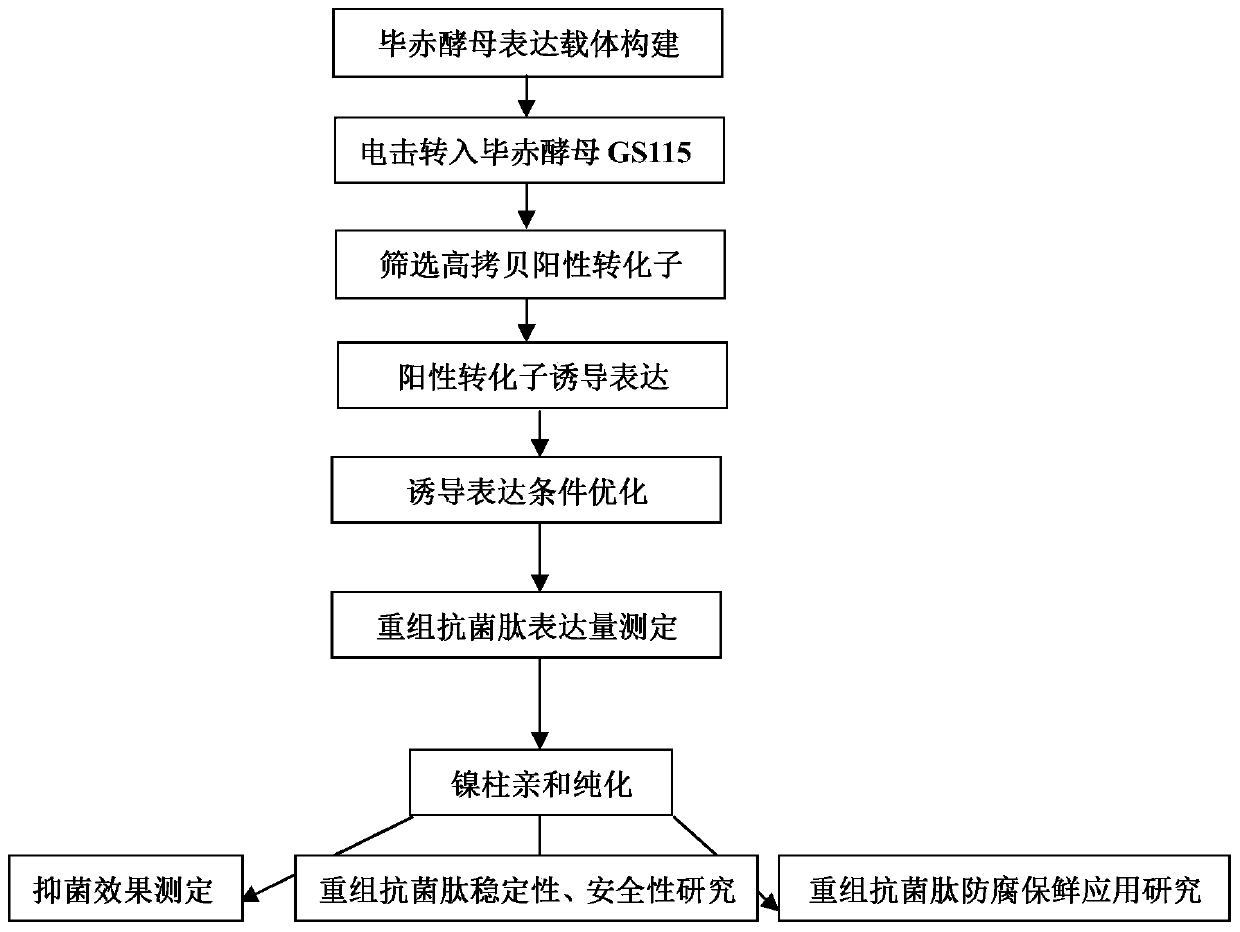
[0076] The preparation of embodiment 1 rMytichitin-CB antimicrobial peptide
[0077] (1) Construction of recombinant expression vector
[0078]Add EcoRI and BamHI restriction sites, start codon, 6×His, and 15 bases of "GATGACGATGACAAG" in sequence at the 5' end of the Mytichitin-CB target gene, and add stop codons, XhoI, KpnI at the 3' end in sequence Restriction sites. Send the gene sequence shown in SEQ ID NO:4 to the company (Suzhou Jinweizhi Biotechnology Co., Ltd.) for synthesis, and connect it to the expression vector pPICZαA through EcoRI and KpnI restriction sites. The connection system is: pPICZαA 20ng, rMytichitin-CB 8ng, T4 ligase 0.8μL, total system 12μL, 4°C, 4h.
[0079] The successfully connected recombinant plasmids were transformed into Escherichia coli competent cells for amplification. Double-digestion verification was performed using restriction endonucleases EcoRI and KpnI.
[0080] Verify the correct strain for sequencing analysis, and the recombinant...
Embodiment 2
[0089] Example 2 rMytichitin-CB antimicrobial peptide performance measurement
[0090] Determination of the minimum inhibitory concentration MIC value - the above-mentioned purified antimicrobial peptide rMytichitin-CB was vacuum freeze-dried into powder, dissolved in sterile Tris-HCl buffer solution (50mM Tris, 100mM NaCl pH 7.4), and the concentrations were respectively Dilute to 2 μg / mL, 4 μg / mL, 6 μg / mL, 12 μg / mL, take 20 μL respectively and add to different wells of a 96-well plate, and make three parallels; set 20 μL of gentamicin at a concentration of 6 μg / mL. The tested bacteria were cultured on a shaker at 37°C to OD 600 1.0-1.5, diluted to 2-5×10 7 CFU / mL, take 100 μL and add to the above wells. Set 100 μL sterile medium plus 20 μL sterile Tris-HCl buffer solution as positive control, 100 μL bacterial solution plus 20 μL sterile Tris-HCl buffer solution as negative control, and do three parallels. After all the addition, seal the 96-well plate with a sealing film ...
Embodiment 3
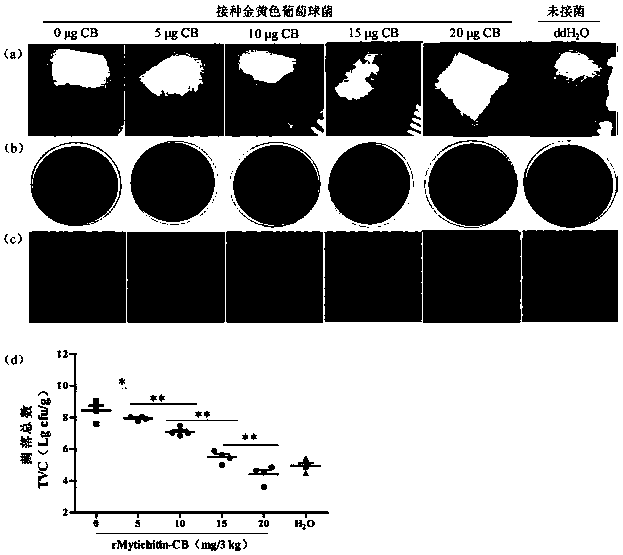
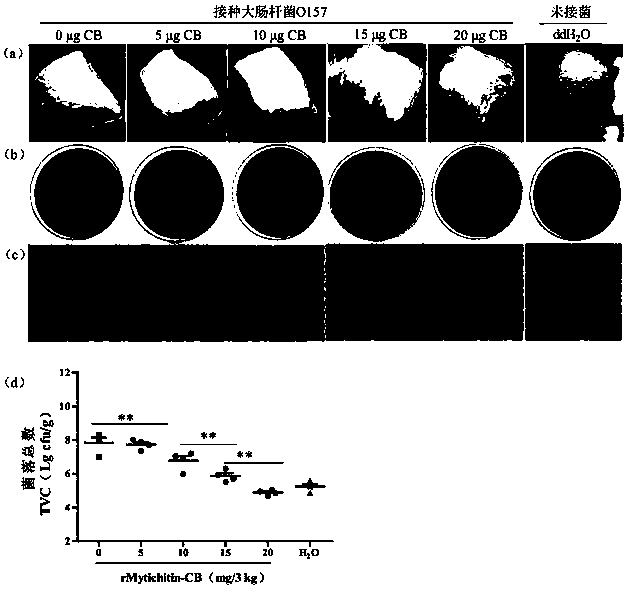
[0108] Embodiment 3 preservation experiment
[0109] The measurement method of each index:
[0110] (1) Sensory evaluation—according to the provisions on sensory requirements in GB 2707-2016 "National Food Safety Standard for Fresh (Frozen) Livestock and Poultry Products", the samples were placed in a white chassis, and the sensory evaluators followed the table below under natural light Score the sample out of 10 points.
[0111] Sensory score sheet
[0112]
[0113]
[0114] (2) The total number of bacterial colonies—according to the method in GB 4789.2-2016 "Determination of the total number of bacterial colonies in food microbiological examination", with appropriate adjustments, the total number of bacterial colonies is determined for meat samples under different storage times. Randomly sample at regular intervals every day, take 5g meat samples from each treatment group and place them in 50mL centrifuge tubes, homogenize them with 25mL normal saline, dilute the hom...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com