Medicine composition containing ceftin cyclodextrin clathrate, and its preparing method
A technology of cyclodextrin inclusion compound and cefuroxime axetil, which is applied in the direction of medical preparations containing active ingredients, medical preparations with non-active ingredients, and pharmaceutical formulas, which can solve the problems of increasing costs and increasing safety risks in clinical application of drugs , increase the environmental protection work of producers and other issues, and achieve the effect of easy dissolution, strong activity and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
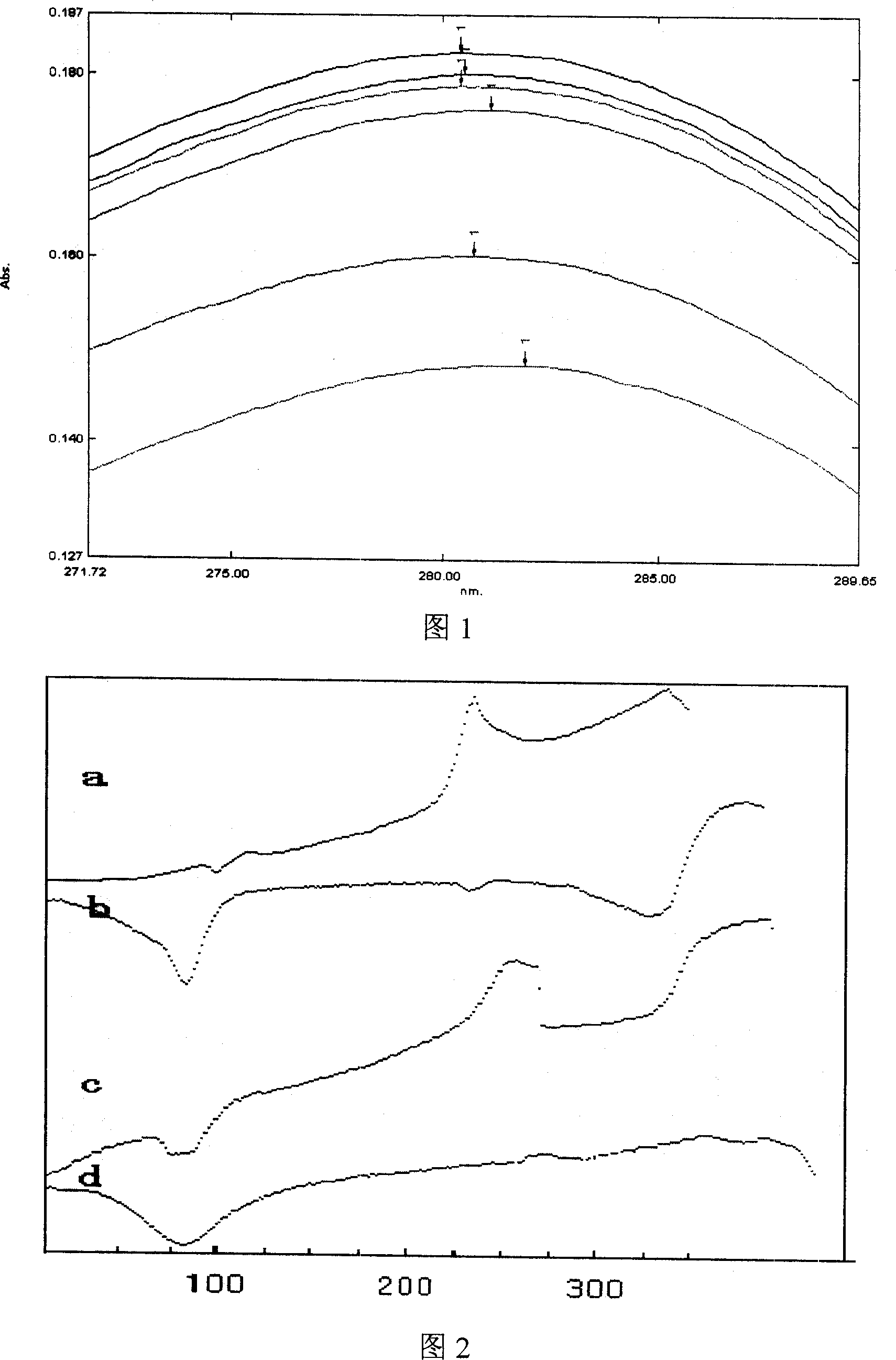
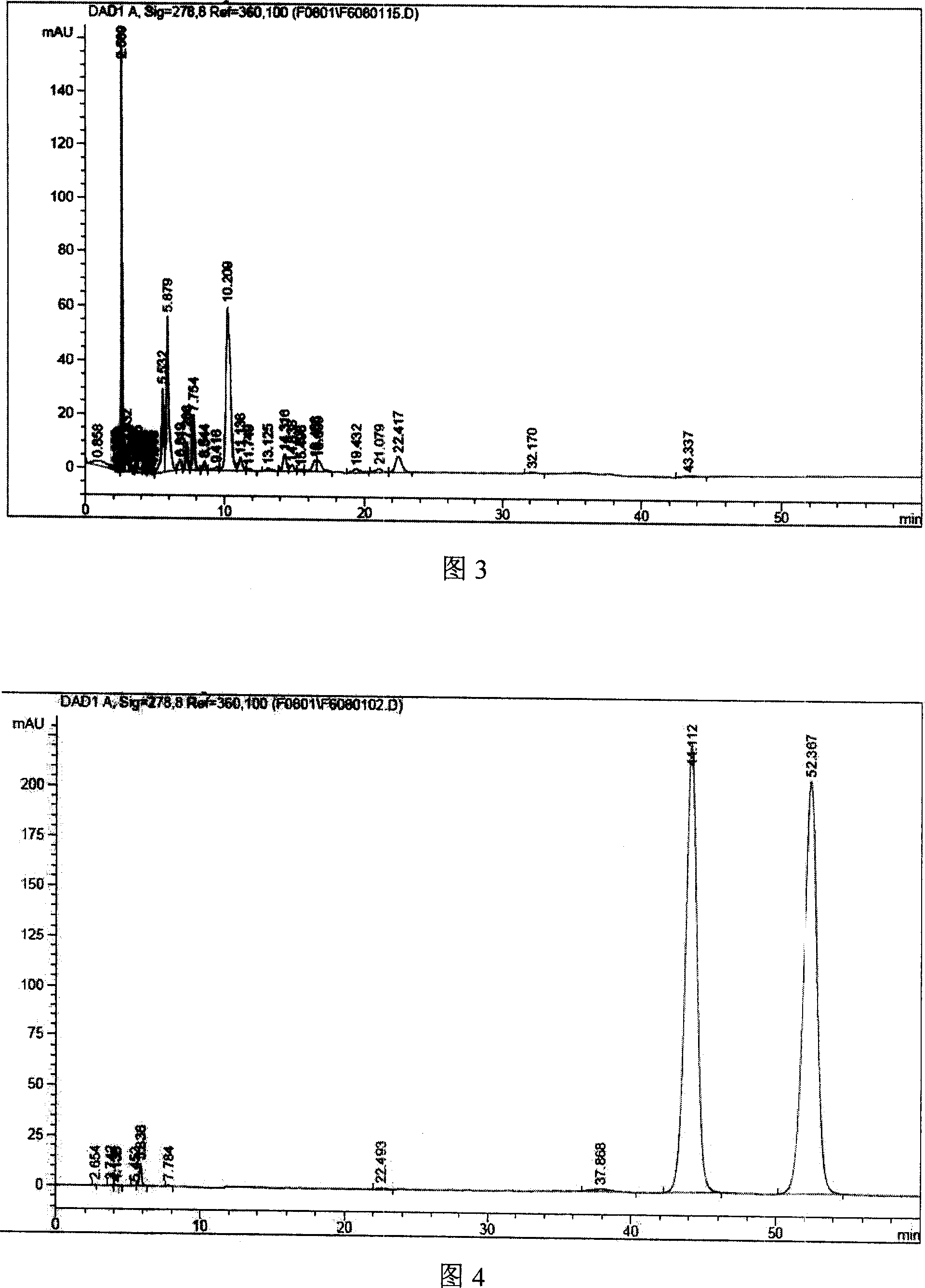
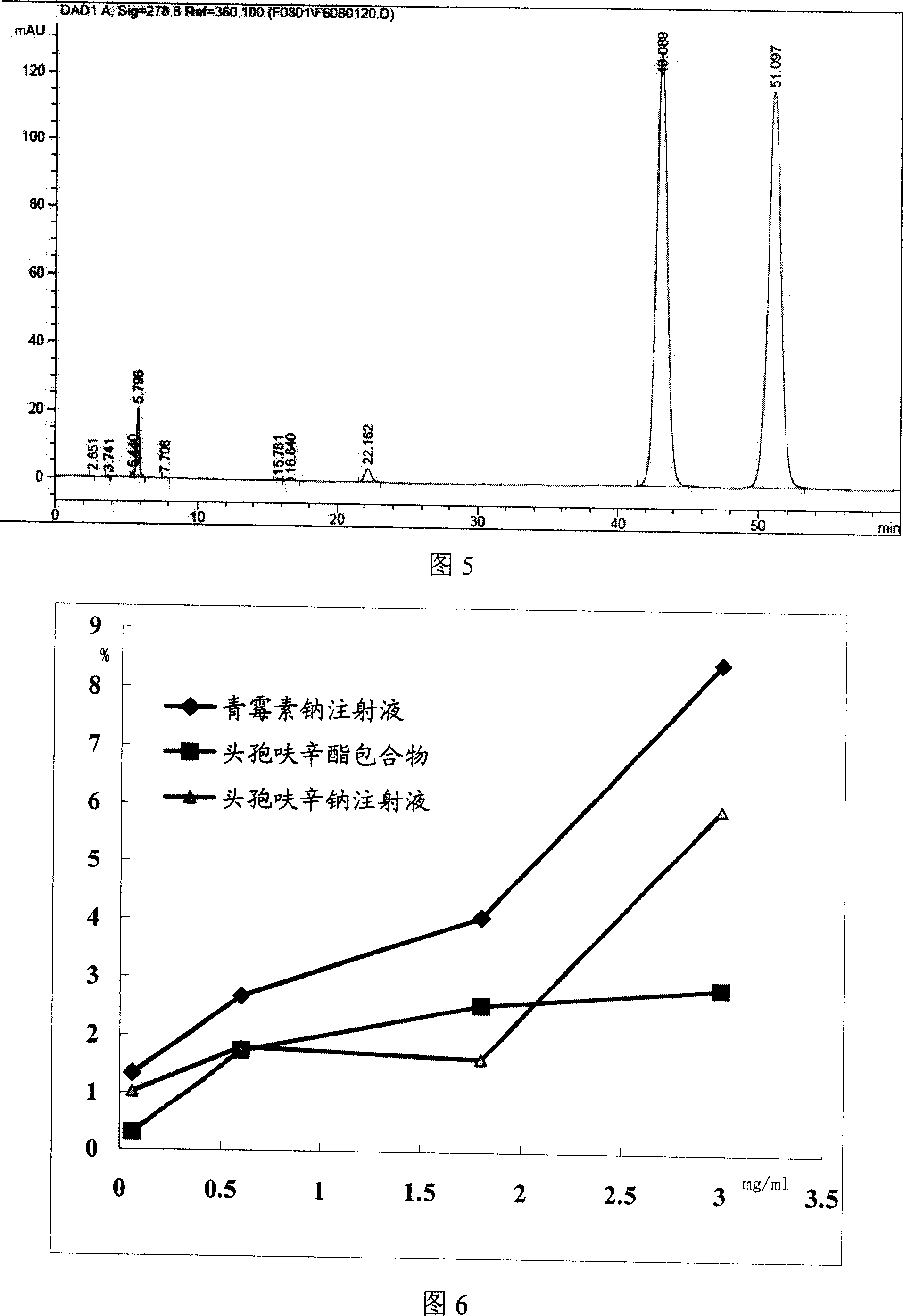
Image
Examples
Embodiment 1
[0078] Mix 300.0g β-cyclodextrin with 300ml pure water, heat, add 60.0g cefuroxime axetil at 50°C, mix well, then add dilute NaHCO drop by drop 3 Solution to pH = 8.0, cooled to room temperature, mixed and ground for 3 hours, then adjusted to pH = 7.0 with dilute HCl acid solution, cooled at 5°C for 24 hours; filtered, washed once with water, and dried under reduced pressure at room temperature to obtain yellow Solid clathrate.
[0079] Mix 360g of solid clathrate (containing 60g of cefuroxime axetil) with 100g of pregelatinized starch, 30g of microcrystalline cellulose, and 10g of croscarmellose sodium, grind them uniformly through a 100-mesh sieve, dry granulate, and prepare The obtained granules are mixed with 2.0g talcum powder and 1.0g magnesium stearate, granulated through a 16-mesh sieve, and compressed to obtain 1000 cefuroxime axetil inclusion tablets, each containing cefuroxime axetil 60mg.
Embodiment 2
[0081] It is basically the same as Example 1, but using 240g β-cyclodextrin and 60g hydroxypropyl-β-cyclodextrin, and after adjusting the dilute HCl acid solution to pH=7.0, remove water under reduced pressure at a temperature of 50°C, after Dry under reduced pressure at room temperature to obtain a yellow solid clathrate.
Embodiment 3
[0083] It is basically the same as Example 1, but using 240g of β-cyclodextrin and 60g of sulfobutyl-β-cyclodextrin, in addition, after the dilute HCl acid solution is adjusted to pH = 7.0, water is removed under reduced pressure at a temperature of 50°C, after Dry under reduced pressure at room temperature to obtain a yellow solid clathrate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com