Crystal forms of vortioxetine organic acid salt and preparation method thereof
A technology of vortioxetine and crystal form, applied in organic chemistry, nervous system diseases, drug combination, etc., can solve the problems of strong corrosion, toxicity, and difficult handling of hydrobromic acid, and achieve improved water solubility and bioavailability Improve and enhance the effect of the application range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
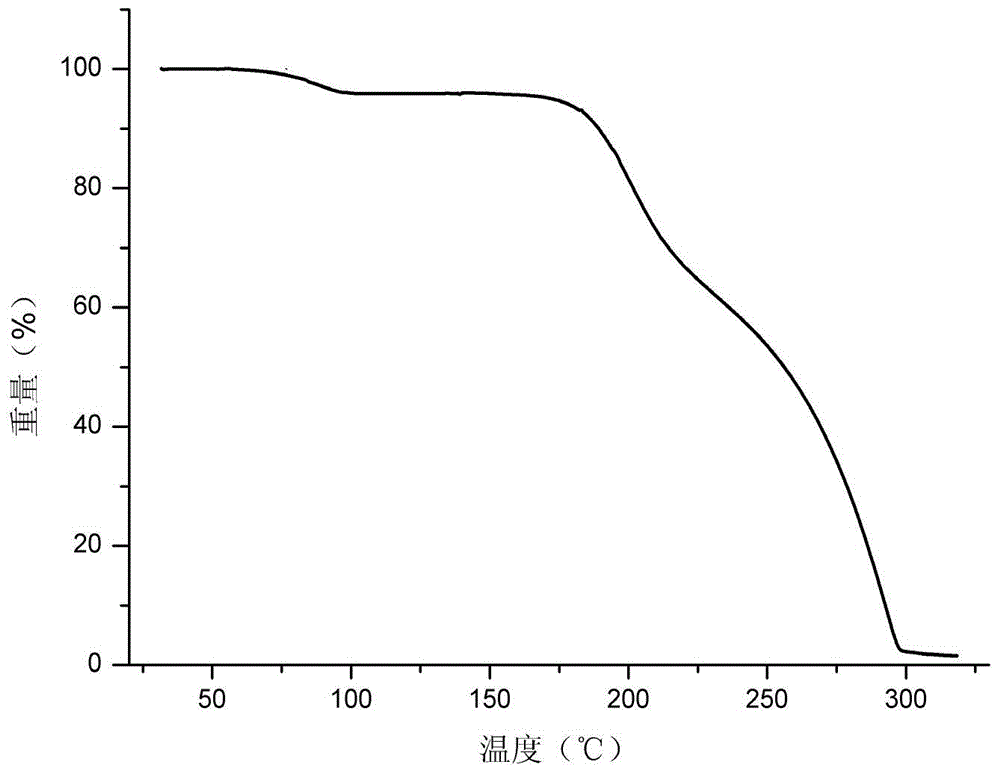
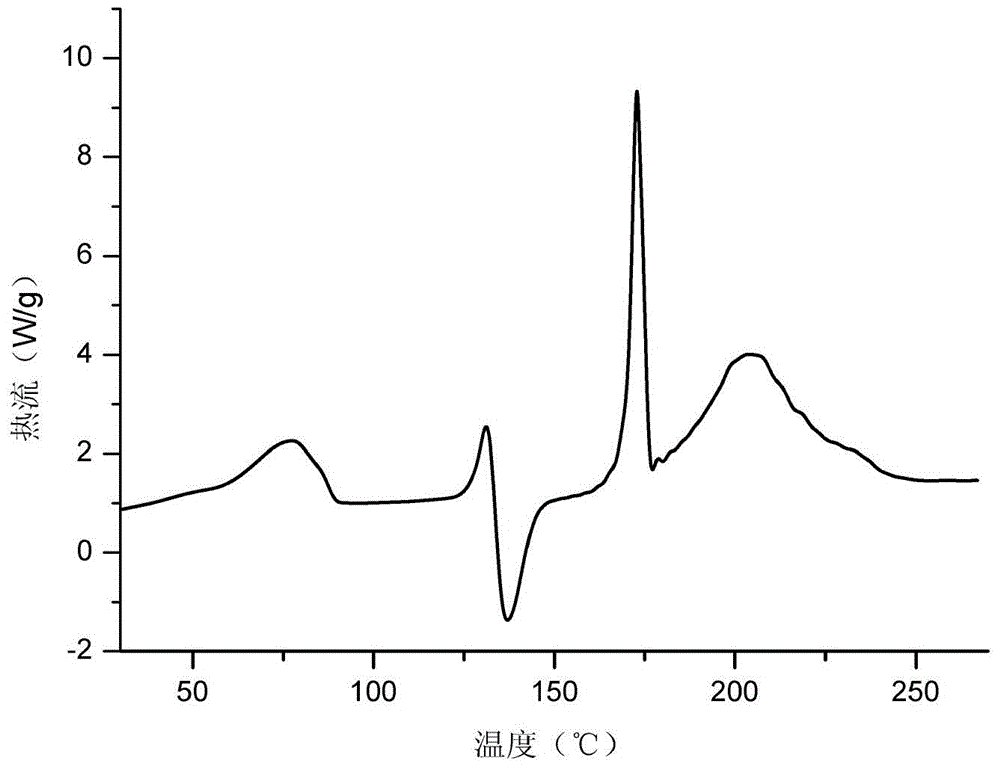
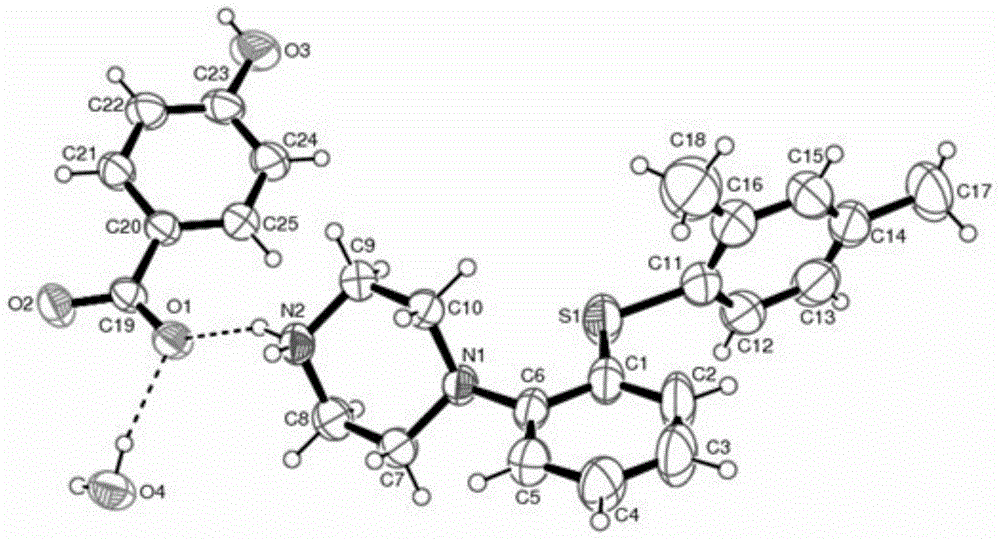
[0101] Embodiment 1: Preparation of vortioxetine p-hydroxybenzoate hydrate
[0102] Add 2.9g of vortioxetine into 15ml of acetone:water (1:1) mixed solvent, and heat to 60°C, add 1.4g of p-hydroxybenzoic acid, stir and dissolve, continue to stir for 2 hours, then cool down naturally to At 30°C, a white solid precipitated, filtered with suction, and dried in a vacuum oven (40-50°C) to obtain Vortioxetine p-hydroxybenzoate hydrate.
Embodiment 2
[0103] Embodiment 2: Preparation of vortioxetine p-hydroxybenzoate hydrate
[0104] Add 15g of vortioxetine to 150ml of ethyl acetate, heat to 50°C to dissolve, dissolve 7.0g of p-hydroxybenzoic acid in 10ml of hot water and add dropwise to the vortioxetine solution, stir for 1 hour, and cool down naturally to At 20°C, white crystals slowly precipitated. After 48 hours, the white solid was obtained by suction filtration, and dried in a vacuum oven (40-50°C) to obtain vortioxetine p-hydroxybenzoate hydrate.
Embodiment 3
[0105] Embodiment 3: Preparation of vortioxetine p-hydroxybenzoate hydrate
[0106] Dissolve 2.9g of vortioxetine in 120ml of 10% ethanol aqueous solution, add 1.4g of p-hydroxybenzoic acid, heat to 70°C, continue to stir for 2h, after dissolving, take it to an ice-water bath for crystallization at 0°C, and slowly precipitate white crystals After 24 hours, the white solid was obtained by suction filtration, and dried in a vacuum oven (40-50° C.) to obtain vortioxetine p-hydroxybenzoate hydrate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com