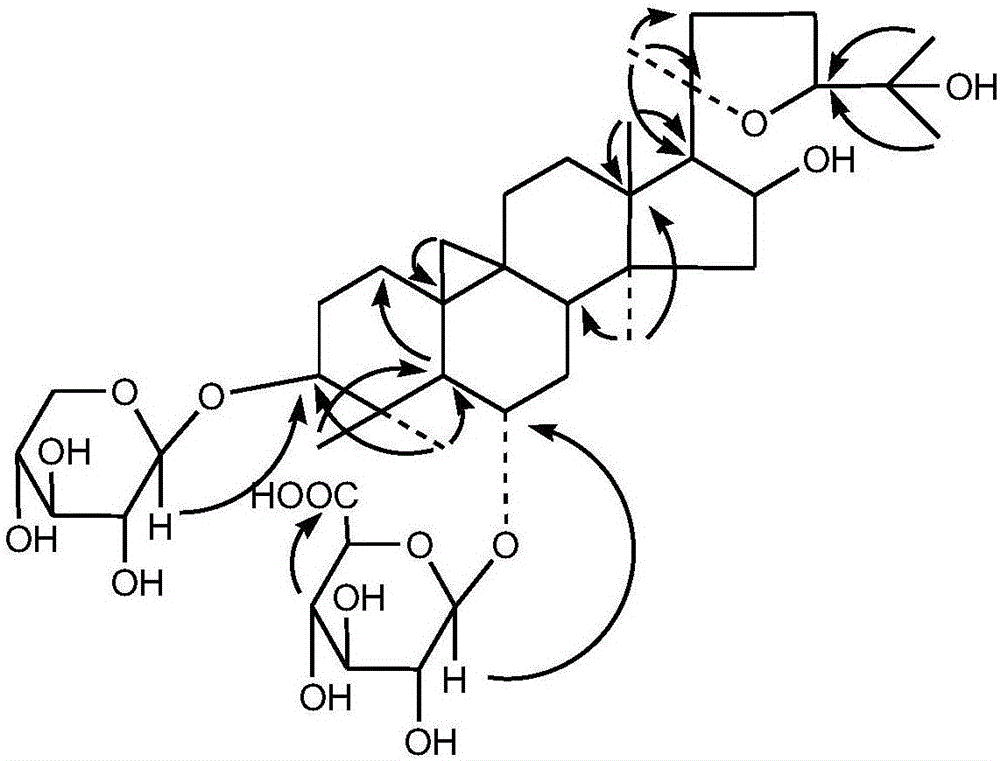
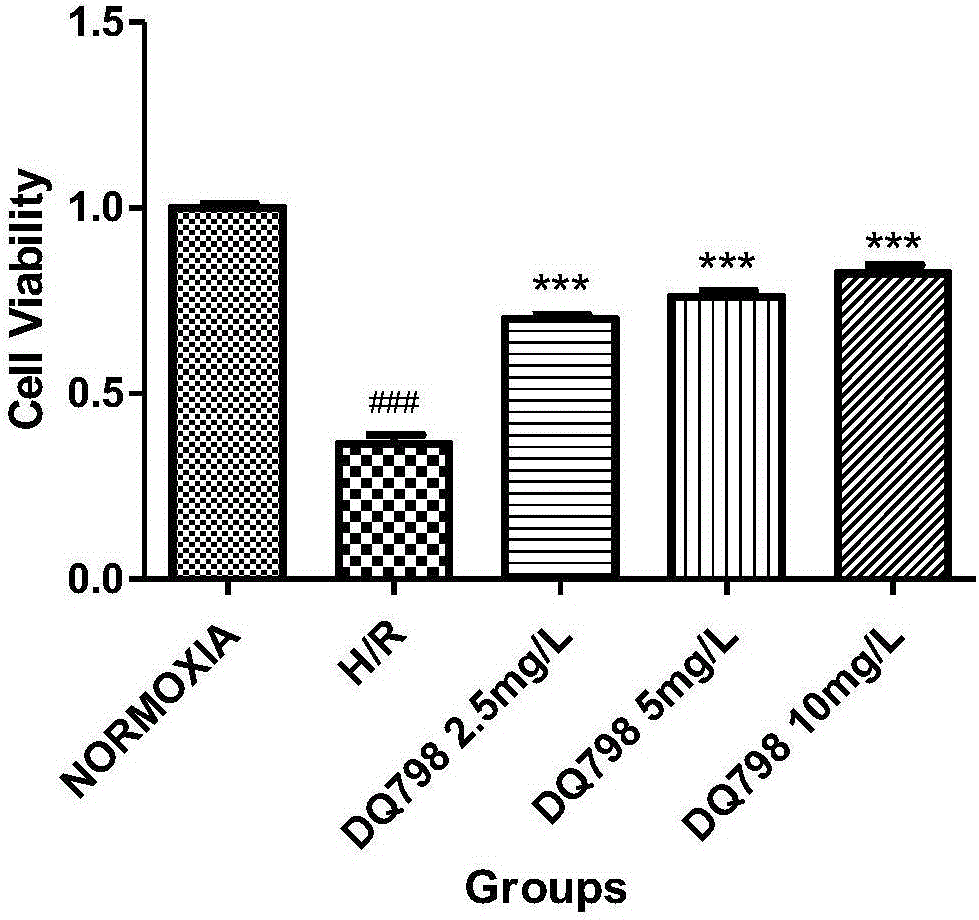
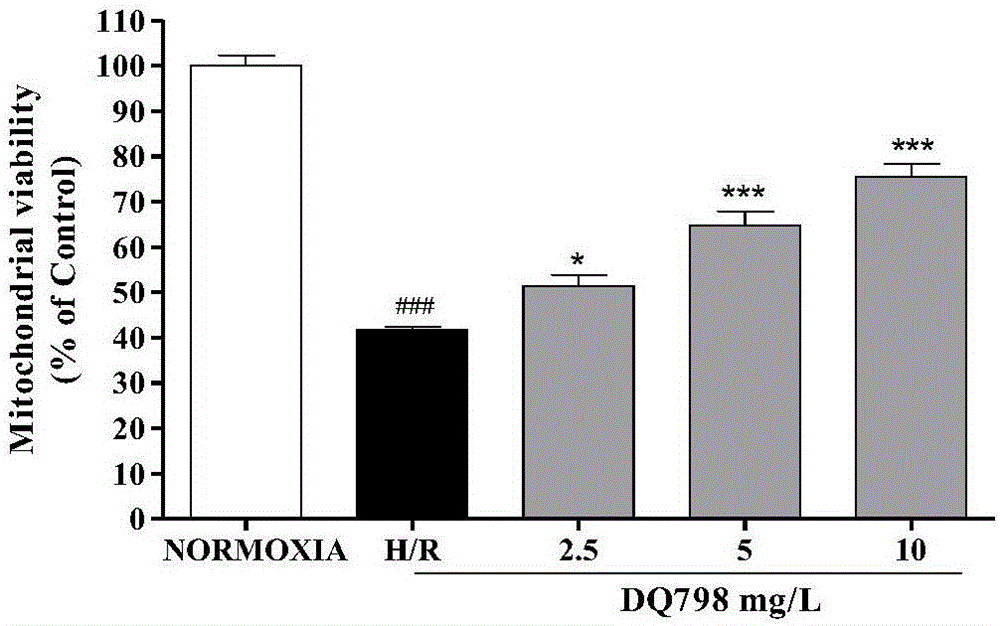
Astragaloside derivative and preparation method and application thereof
A technology for a drug and methylamine, applied in the field of medicine, can solve the problems of many side reactions in the synthesis route, no prodrug properties, and two sugar chains are easy to fall off, so as to achieve improved druggability, less side reactions, and increased water solubility. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] This embodiment provides the preparation method and structural identification of the compound having the structure of formula 1 described in claim 1:
[0028] (1) Compound preparation: Take 100 mg of astragaloside IV, add it to 500 mL of 10% pyridine, add 25 mg of sodium bromide, 5 mg of 2,2,6,6-tetramethylpiperidine-1-oxyl free radical, 1mL of sodium hypochlorite, reacted at 0°C for 120min, filtered, adjusted the pH of the filtrate to 3 with concentrated hydrochloric acid, concentrated to 20mL after the solid precipitated, separated the solid from the liquid, dissolved the solid with methanol and concentrated to dryness to obtain 89.7mg of white powder. A compound having the structure of formula 1 (HPLC purity 98.8%).
[0029] (2) Structural identification:
[0030] White amorphous powder; after TLC development, spray 5% concentrated sulfuric acid / ethanol to show purple; (c, 1.0, MeOH); IR(KBr): Vmax: 3417, 2968, 1732, 1621, 1378, 1156, 1066, 1045cm -1 . HR-ESI-MS...
Embodiment 2
[0039] This embodiment provides the preparation method of the compound having the structure of formula 1 described in claim 1. Compared with Example 1, the difference is that the ratio of solvent and reagent is adjusted:
[0040] Take 100mg of astragaloside IV, add it to 50mL of water, add 25mg of sodium bromide, 10mg of 2,2,6,6-tetramethylpiperidine-1-oxyl free radical, 0.25mL of sodium hypochlorite, and react at 20°C 120min, filter, the filtrate is adjusted to pH2 with concentrated hydrochloric acid, after the solid is precipitated, concentrate to 10mL, separate the solid and liquid, dissolve the solid with ethanol and transfer, concentrate to dryness, and obtain 91.3mg of white powder which is the compound having the structure of formula 1 (HPLC purity 99.1%).
Embodiment 3
[0042] This embodiment provides the preparation method of the compound having the structure of formula 1 described in claim 1. Compared with Example 1, the difference is that the ratio of solvent and reagent is adjusted:
[0043] Take 100mg of astragaloside IV, add it into 10mL of 10% DMF, add 20mg of sodium bromide, 8mg of 2,2,6,6-tetramethylpiperidine-1-oxyl free radical, 0.20mL of sodium hypochlorite in turn, React at ℃ for 120min, filter, adjust the pH of the filtrate to 3 with concentrated hydrochloric acid, concentrate to 10mL after the solid precipitates, separate the solid from the liquid, dissolve the solid with ethanol and transfer, concentrate to dryness, and obtain 82.7mg of white powder which is the compound with the structure of formula 1 Compound (HPLC purity 97.9%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com