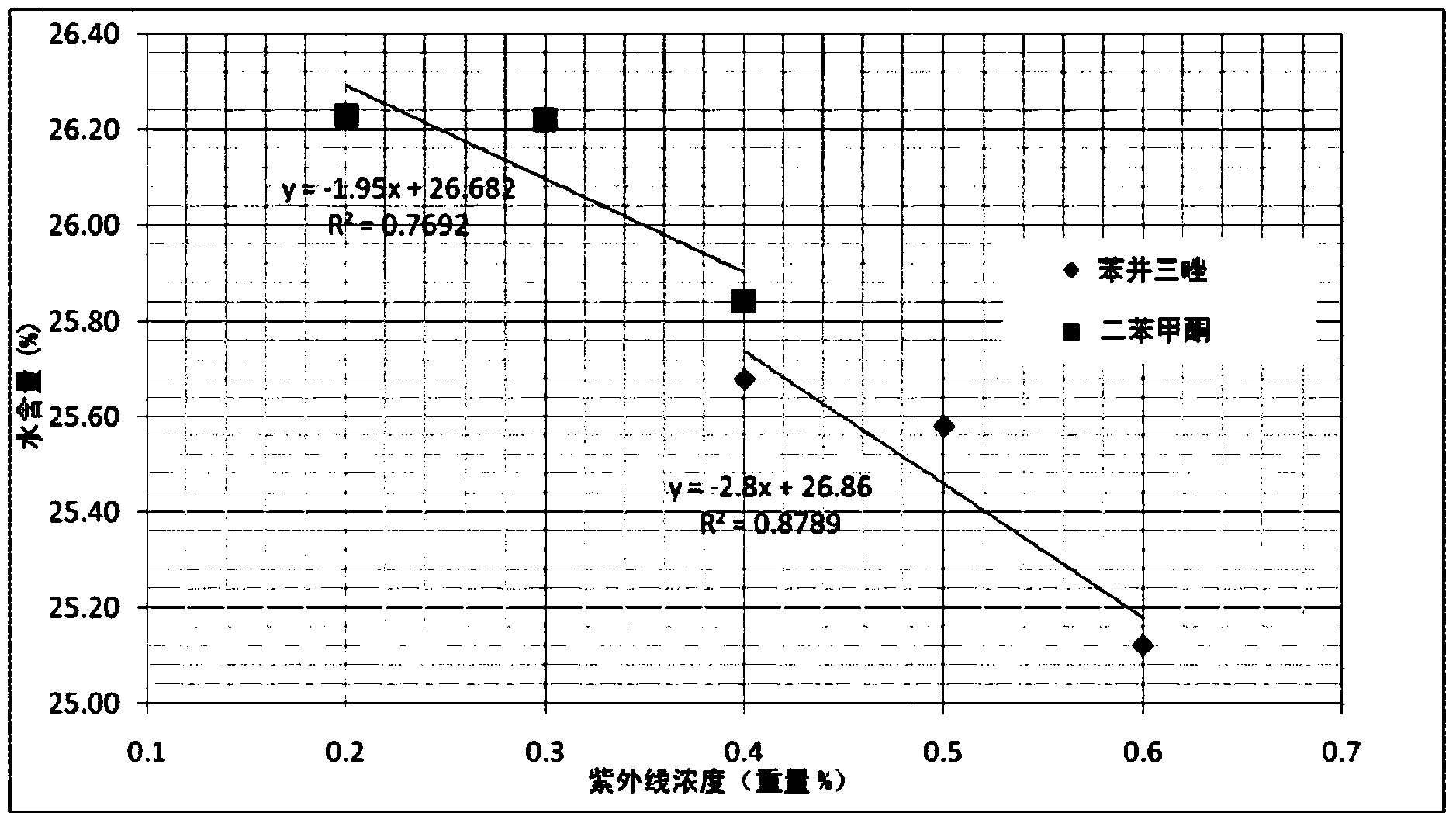
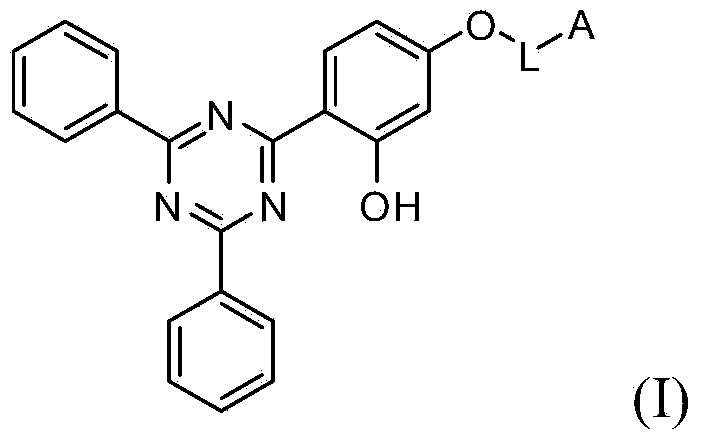
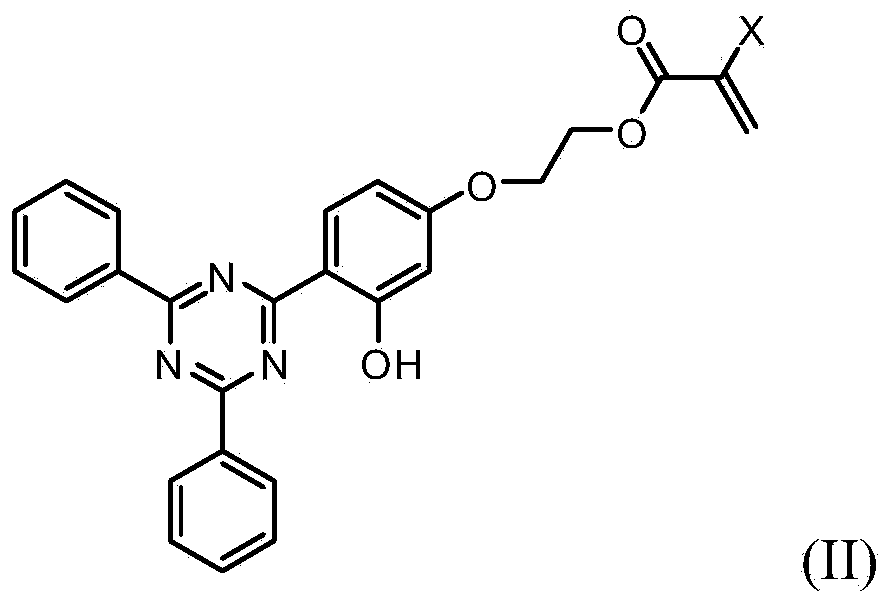
Ultraviolet light absorbing materials for intraocular lens and uses thereof
A technology of intraocular lens and lens, applied in the direction of intraocular lens, prosthesis, instrument, etc., can solve the problem of not being able to provide UV absorption, etc., and achieve the effect of increasing water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Embodiment 1: the synthesis of HPTZ
[0064]
[0065] 10.8g (31.7mmol) 2-(2,4-dihydroxyphenyl)-4,6-diphenyl-1,3,5-triazine, 5.8g (39.1mmol) 2-chloroethyl methacrylate A solution of the ester and 5.8 g (42.0 mmol) of anhydrous potassium carbonate in 200 ml of DMSO was heated in an oil bath preheated to 82°C for 17.5 hours. The final bath temperature was 87°C. In two systems (silica gel, hexane:acetone::3:1 (v / v) and CH 2 Cl 2 ) showed no starting material. After cooling to room temperature, 3100 ml DI water was added. A thick slurry was obtained at first, which thinned with each addition of water. A significant exotherm was observed on the first two additions of water, but on the third addition, the exotherm was minimal. Transfer the contents of the flask to a 1 L separatory funnel and use 100 ml of DI water to rinse the flask. 2×200ml CH for aqueous suspension 2 Cl 2 and finally with 100ml CH 2 Cl 2 Extraction and the combined organic extracts were concent...
Embodiment 2
[0066] Example 2: Hydrophobic polymers suitable for use in IOLs 1
[0067] Mix 35.0g EOEMA with 2.0g HEA, 2.0g LMA, 1.0g GMA, 0.040g HPTZ, 0.021g2,2'-azobis(2,4-dimethylvaleronitrile), 0.08g2,2'-azo Bis(2-methylbutyronitrile) and 1.1 g TMPTMA were mixed. The mixture was degassed while vigorous stirring was applied. The mixture was dispensed into molds, polymerized at 70°C for 8 hours, and post-cured at 95°C for 10 hours. Allow the mold to cool to room temperature. The mold was opened and the polymer disk was removed and inspected.
Embodiment 3
[0068] Example 3: Hydrophobic polymers suitable for use in IOLs 2
[0069] Mix 35.0g EOEMA with 2.0g HEA, 2.0g LMA, 1.0g GMA, 0.050g HPTZ, 0.021g2,2'-azobis(2,4-dimethylvaleronitrile), 0.08g2,2'-azo Bis(2-methylbutyronitrile) and 1.1 g TMPTMA were mixed. The mixture was degassed while vigorous stirring was applied. The mixture was dispensed into molds, polymerized at 70°C for 8 hours, and post-cured at 95°C for 10 hours. Allow the mold to cool to room temperature. The mold was opened and the polymer disk was removed and inspected.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com