Biodegradable redox sensitive polymer and preparation method and application thereof
A polymer and biological technology, applied in the field of pharmaceutical preparations and polymer chemistry, can solve the problems of lack of pertinence, worsening side effects, and limited effects, and achieve the effect of good inhibition, reduction of toxic and side effects, and enhancement of targeting.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1 Preparation of hyaluronic acid-S-S-vitamin E succinate polymer (HA-SS-VES)
[0028] (1) Preparation method
[0029]1. Dissolve vitamin E succinate (1.06g, 2mmol) in 50mL of dichloromethane, stir in a 250mL round-bottomed flask until completely dissolved, add EDC (13mmol) and HOBT (13mmol) in an ice bath, and keep it away from light at room temperature Stir overnight. Cystamine dihydrochloride (1.35 g, 6 mmol) was added to the resulting reaction solution, and 10 mL of methanol was added to aid dissolution, triethylamine was added to adjust the pH to 7-8, and stirred for 24 h. The obtained product was washed with 1 mol / L NaHCO 3 The aqueous solution was washed, the organic layer was dried by adding anhydrous magnesium sulfate, filtered, the dichloromethane was removed by rotary evaporation under reduced pressure at 40° C., and vacuum-dried to obtain 0.944 g of vitamin E succinate derivative with a yield of 71.1%. MS(ESI)m / z(%):665.4[M+H] + ; IR (KBr, cm-1): 3...
Embodiment 2
[0031] Example 2 HA-SS-VES drug-loaded nanomicelle
[0032] (1) Preparation of drug-loaded nanomicelles
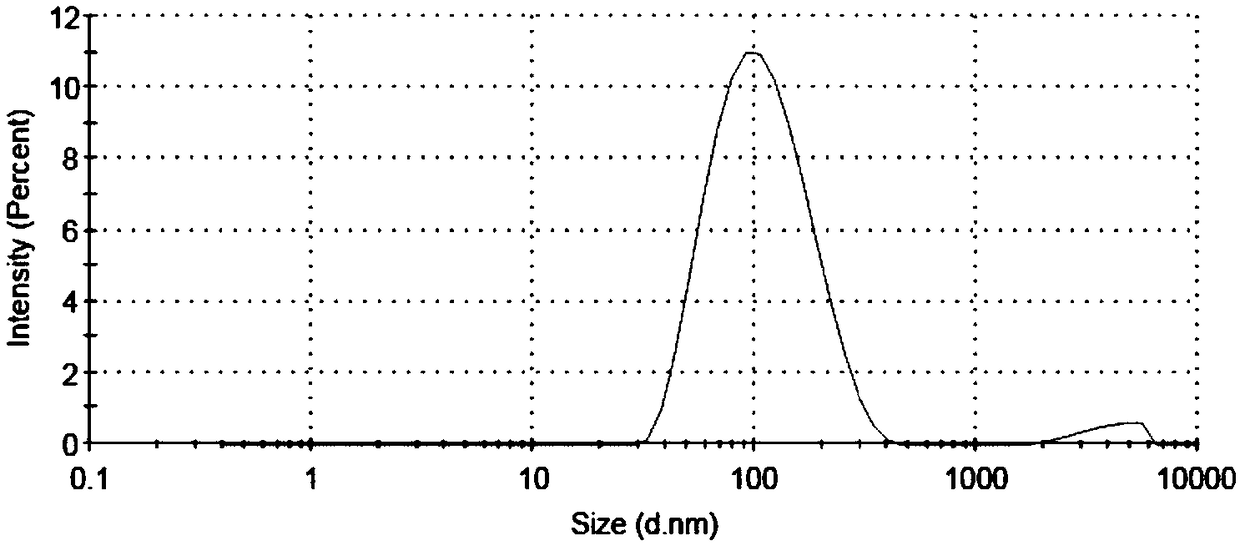
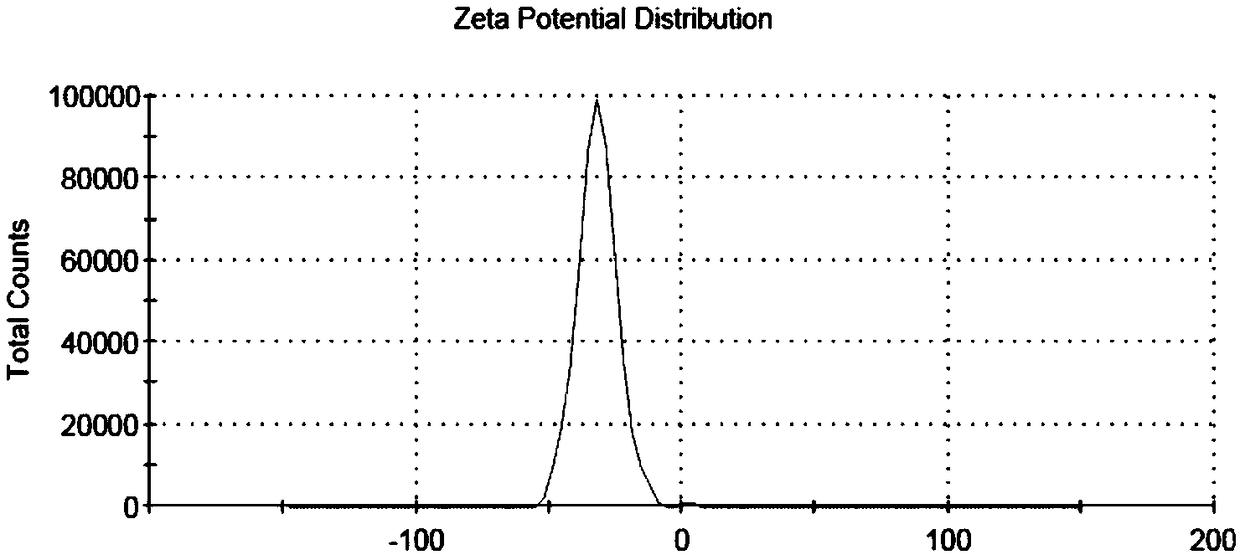
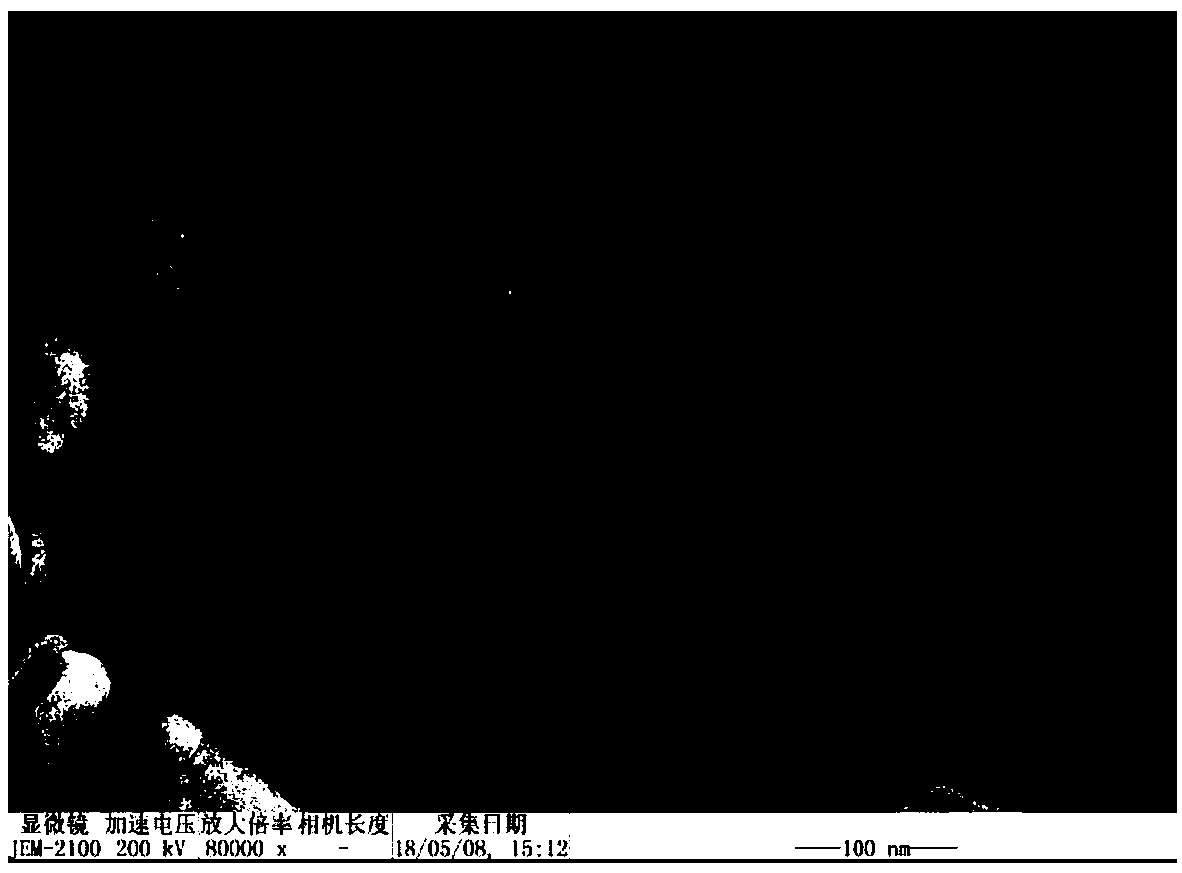
[0033] Take 20mg gambogic acid and 50mg polymer HA-SS-VES, put them in a 250mL eggplant-shaped bottle, add 10mL dichloromethane to dissolve. Dichloromethane was removed by rotary evaporation at 40°C under reduced pressure to obtain a yellow drug film, then 50 mL of distilled water was added, hydrated in a water bath at 65°C for 4 hours, and shaken well. In an ice bath, ultrasonically treat the probe (ultrasonic cell breaker) 3 times, 2min each time, with an ultrasonic power of 225W, and pass through a 0.45μm microporous membrane to obtain the drug-loaded nano-particles loaded with gambogic acid in the HA-SS-VES polymer. micellar solution.
[0034] (2) Investigation of drug-loaded nanomicelle water bath time
[0035] According to the preparation method described in (1), change the water bath time, and hydrate in a water bath at 65°C for 2, 4, 6, and 8 hours respectively....
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com