Arbutin/hydroxypropyl-beta-cyclodextrin inclusion compound and preparation method thereof
A cyclodextrin inclusion complex, hydroxypropyl technology, applied in the chemical field, can solve the problems of skin irritation, allergic reaction, poor water solubility and stability of arbutin, improve thermal stability, widen the scope of application, The effect of improving water solubility and thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
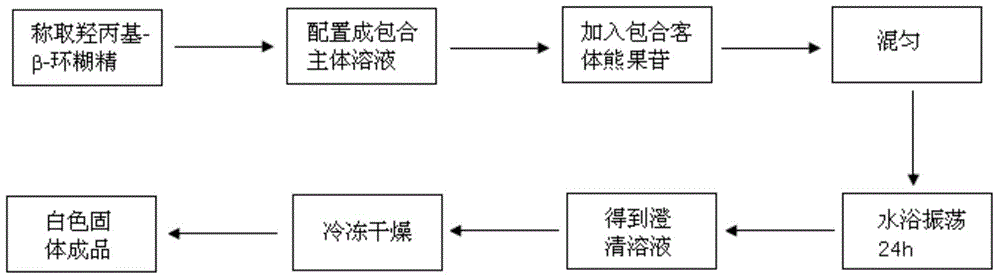
[0032] A preparation method of arbutin / hydroxypropyl-β-cyclodextrin inclusion compound, which comprises the following steps:
[0033] (1) Weigh 2.920g (2mmol) of hydroxypropyl-β-cyclodextrin (HP-β-CD) and dissolve it in 100mL of distilled water to prepare a 0.02mol / L hydroxypropyl-β-cyclodextrin solution;
[0034] (2) According to the ratio of the amount of hydroxypropyl-β-cyclodextrin to arbutin as 2:1, weigh 0.272g (1mmol) of arbutin;
[0035] (3) Add the arbutin weighed in step (2) to the hydroxypropyl-β-cyclodextrin solution prepared in step (1) and mix to prepare a mixed solution A;
[0036] (4) Place the mixed solution A in step (3) in a sealed Erlenmeyer flask and shake in a 60°C water bath for 24 hours to obtain a clear solution B;
[0037] (5) Pour the clear solution B in step (4) into a fresh-keeping box and freeze-dry to obtain a white solid, which is an arbutin / hydroxypropyl-β-cyclodextrin inclusion compound.
Embodiment 2
[0039] A preparation method of arbutin / hydroxypropyl-β-cyclodextrin inclusion compound, which comprises the following steps:
[0040] (1) Weigh 2.920g (2mmol) of hydroxypropyl-β-cyclodextrin (HP-β-CD) and dissolve it in 100mL of distilled water to prepare a 0.02mol / L hydroxypropyl-β-cyclodextrin solution;
[0041] (2) According to the ratio of the amount of hydroxypropyl-β-cyclodextrin to arbutin as 1:1, weigh 0.554g (2mmol) of arbutin;
[0042] (3) Add the arbutin weighed in step (2) to the hydroxypropyl-β-cyclodextrin solution prepared in step (1) and mix to prepare a mixed solution A;
[0043] (4) Place the mixed solution A in step (3) in a sealed Erlenmeyer flask and shake in a 60°C water bath for 24 hours to obtain a clear solution B;
[0044] (5) Pour the clear solution B in step (4) into a fresh-keeping box and freeze-dry to obtain a white solid, which is an arbutin / hydroxypropyl-β-cyclodextrin inclusion compound.
Embodiment 3
[0046] A preparation method of arbutin / hydroxypropyl-β-cyclodextrin inclusion compound, which comprises the following steps:
[0047] (1) Weigh 2.920g (2mmol) of hydroxypropyl-β-cyclodextrin (HP-β-CD) and dissolve it in 100mL of distilled water to prepare a 0.02mol / L hydroxypropyl-β-cyclodextrin solution;
[0048] (2) According to the ratio of the amount of hydroxypropyl-β-cyclodextrin to arbutin as 2:3, weigh 0.816g (3mmol) of arbutin;
[0049] (3) Add the arbutin weighed in step (2) to the hydroxypropyl-β-cyclodextrin solution prepared in step (1) and mix to prepare a mixed solution A;
[0050] (4) Place the mixed solution A in step (3) in a sealed Erlenmeyer flask and shake in a 60°C water bath for 24 hours to obtain a clear solution B;
[0051] (5) Pour the clear solution B in step (4) into a fresh-keeping box and freeze-dry to obtain a white solid, which is an arbutin / hydroxypropyl-β-cyclodextrin inclusion compound.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com