Clarified propofol injection and preparation method thereof
A technology of injection and propofol, which is applied in the field of medicine, can solve the problems of long preparation time, high concentration of free propofol, and ineffective pain effect, and can reduce the risk of injection pain, small hemolysis, and The effect of low dosage of excipients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~8
[0067] At room temperature, take the prescribed amount of sulfobutyl ether-β-cyclodextrin and the corresponding prescribed amount of sodium bicarbonate, add 50 mL of water for injection, stir and dissolve, pass nitrogen through the solution to remove dissolved oxygen, and add 1000mg of propofol, stir for 2-6 hours, adjust the pH value to 6-9, add water to 100mL, stir evenly, sterilize by 0.22μm filter, put it into a transparent sterile ampoule bottle, and seal it with sterile nitrogen to exhaust the air. Store at 25°C. Wherein in each embodiment the consumption of raw material and the outward appearance of the injection that obtains and the concentration of free propofol are shown in Table 1.
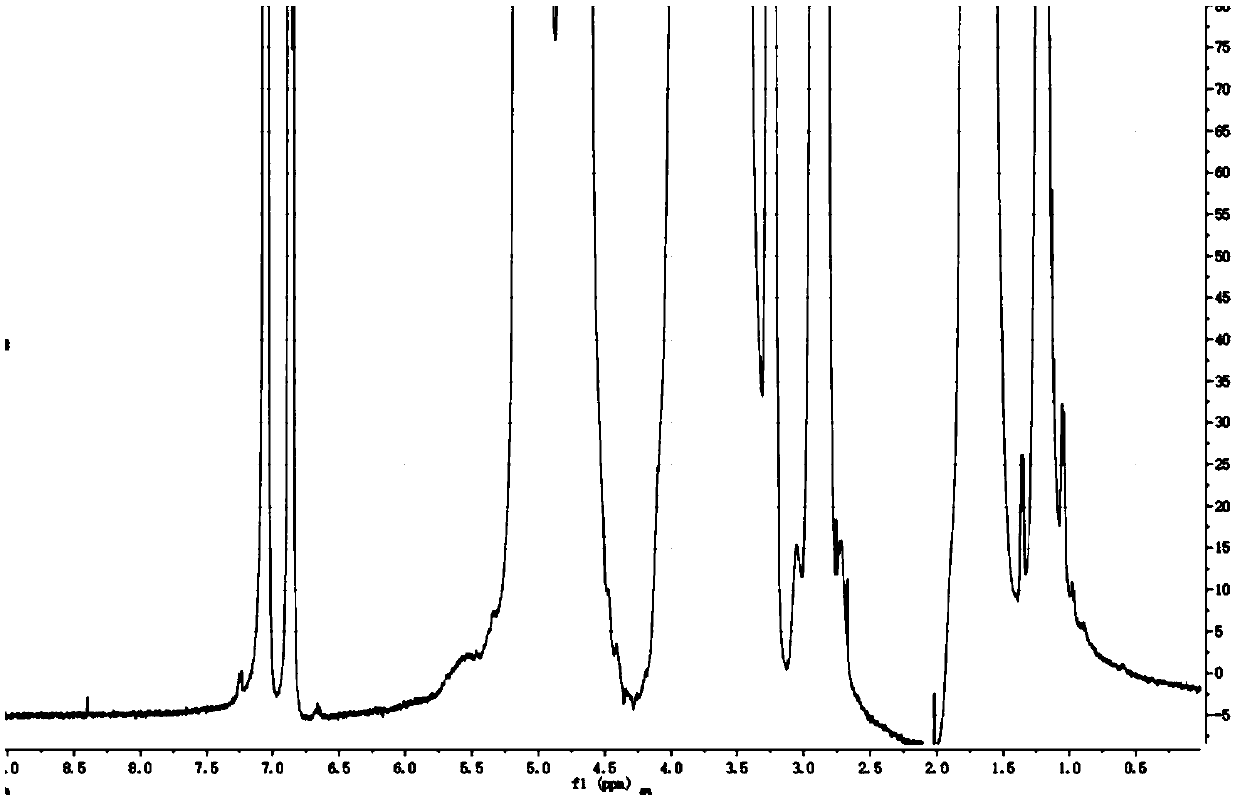
[0068] figure 1 The magnified figure of the proton nuclear magnetic resonance spectrum of the sample prepared according to Example 2 is listed.
Embodiment 9~10
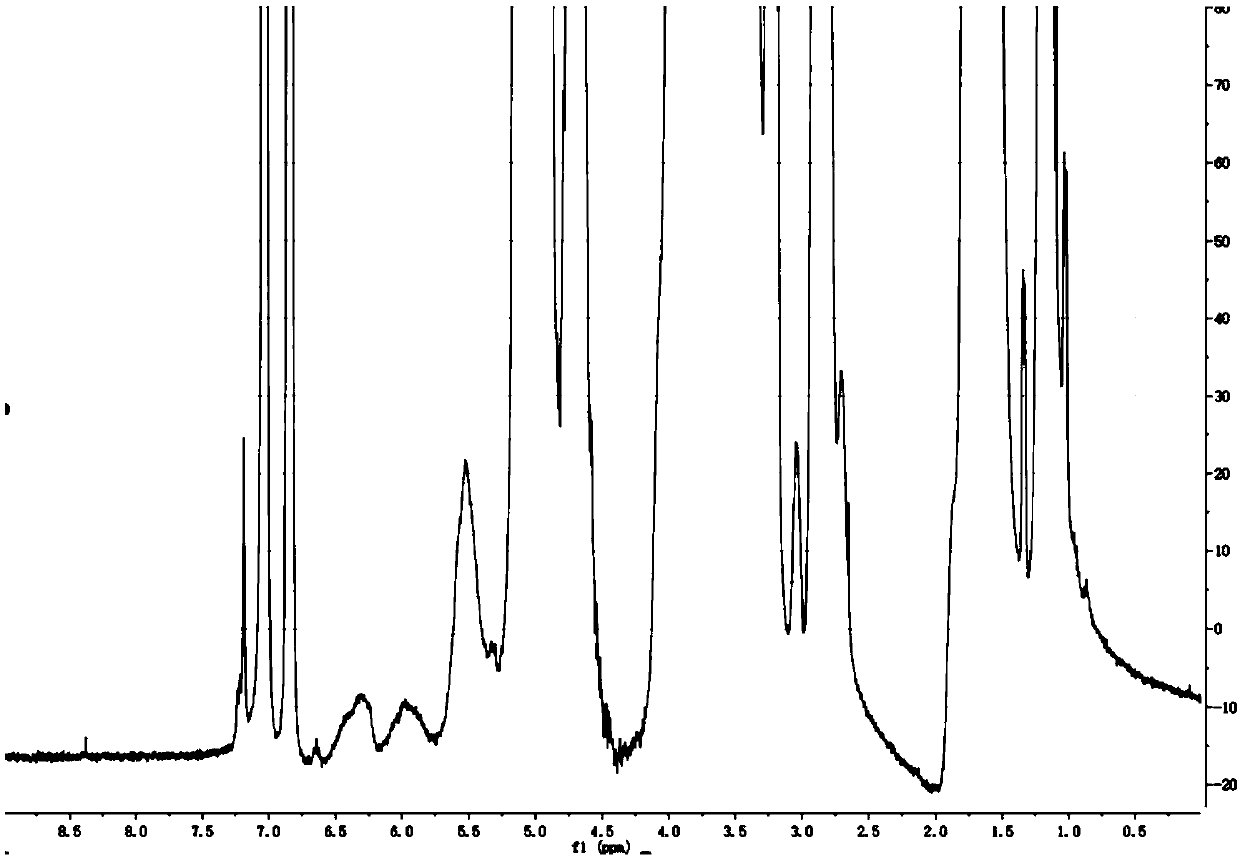
[0070] Examples 9-10 were prepared with reference to the patents of Cydex Corporation, specifically referring to Examples 1 and 2 of US7034013. figure 2 The magnified figure of the proton nuclear magnetic resonance spectrum of the sample prepared according to Example 9 is listed.
[0071] Wherein in each embodiment the consumption of raw material and the outward appearance of the injection that obtains and the concentration of free propofol are shown in Table 1.
[0072] Table 1
[0073]
[0074]
[0075] From Examples 1 to 10, it can be seen that when sodium bicarbonate is higher than 1% (w / v), the preparation is slightly yellow and has poor stability, and when sodium bicarbonate is lower than 0.01% (w / v), the preparation is milky. Comparing Example 9 and Example 10, increasing the concentration of sulfobutyl ether-β-cyclodextrin cannot significantly reduce the concentration of free propofol.
[0076] In Examples 8-10 that do not contain sodium bicarbonate, when the ...
Embodiment 11
[0078] At room temperature, take 18.0g of sulfobutyl ether-β-cyclodextrin, 100mg of sodium citrate, add 50mL of water for injection, stir and dissolve, pass nitrogen through the solution to exclude dissolved oxygen, and add 1000mg of propofol under nitrogen atmosphere Phenol, stir for 2-6 hours, adjust the pH value to 6-9, add water to 100mL, stir evenly, sterilize by 0.22μm filter, put it into a transparent sterile ampoule, seal it with sterile nitrogen to exhaust the air, and store it at 25°C After storage, the measured free propofol concentration was 37.3 μg / mL.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com