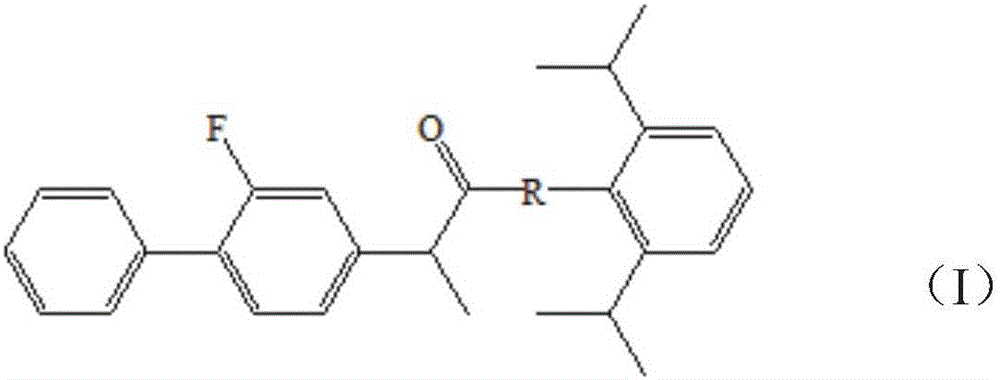
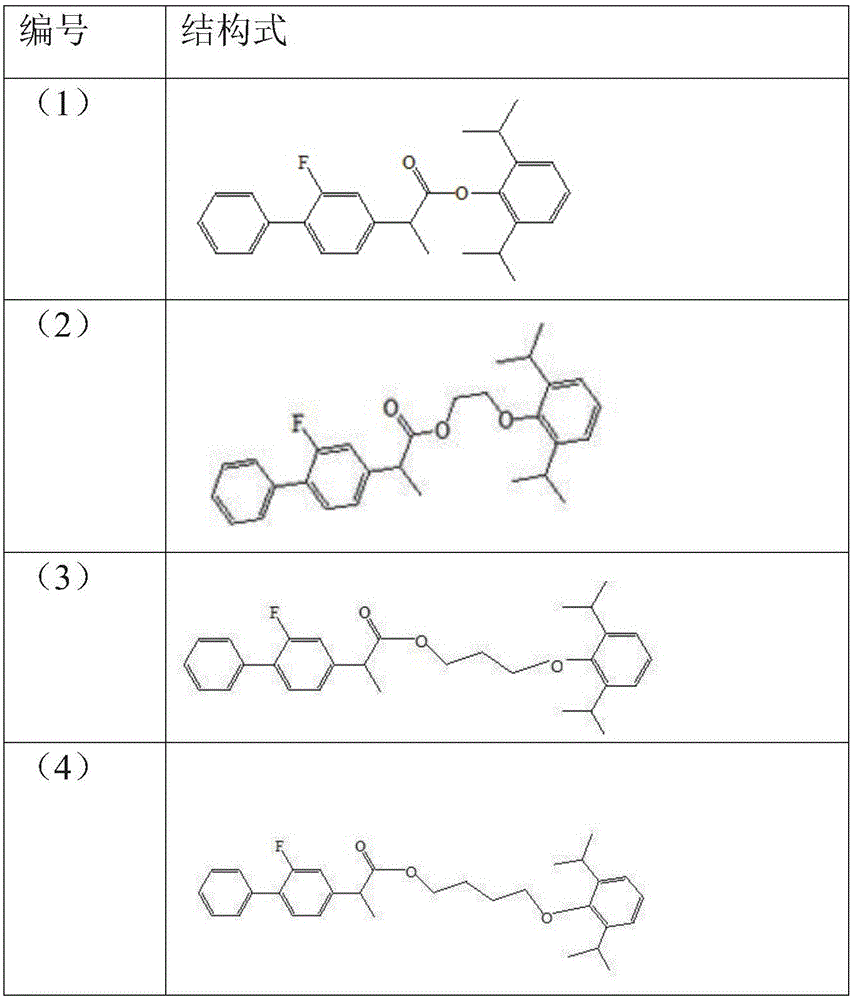
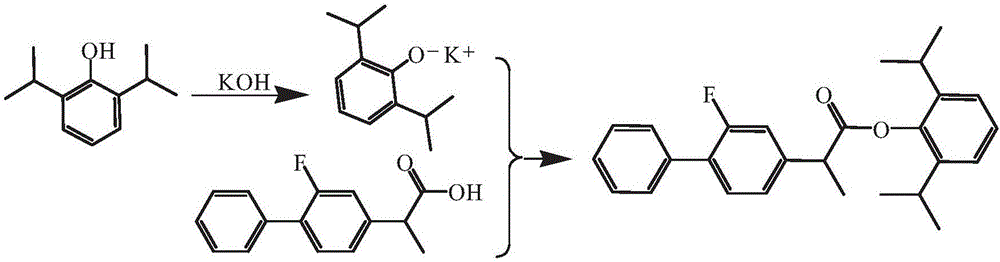
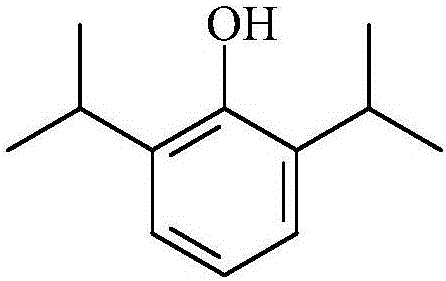
Patents
Literature
112 results about "Propofolum" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
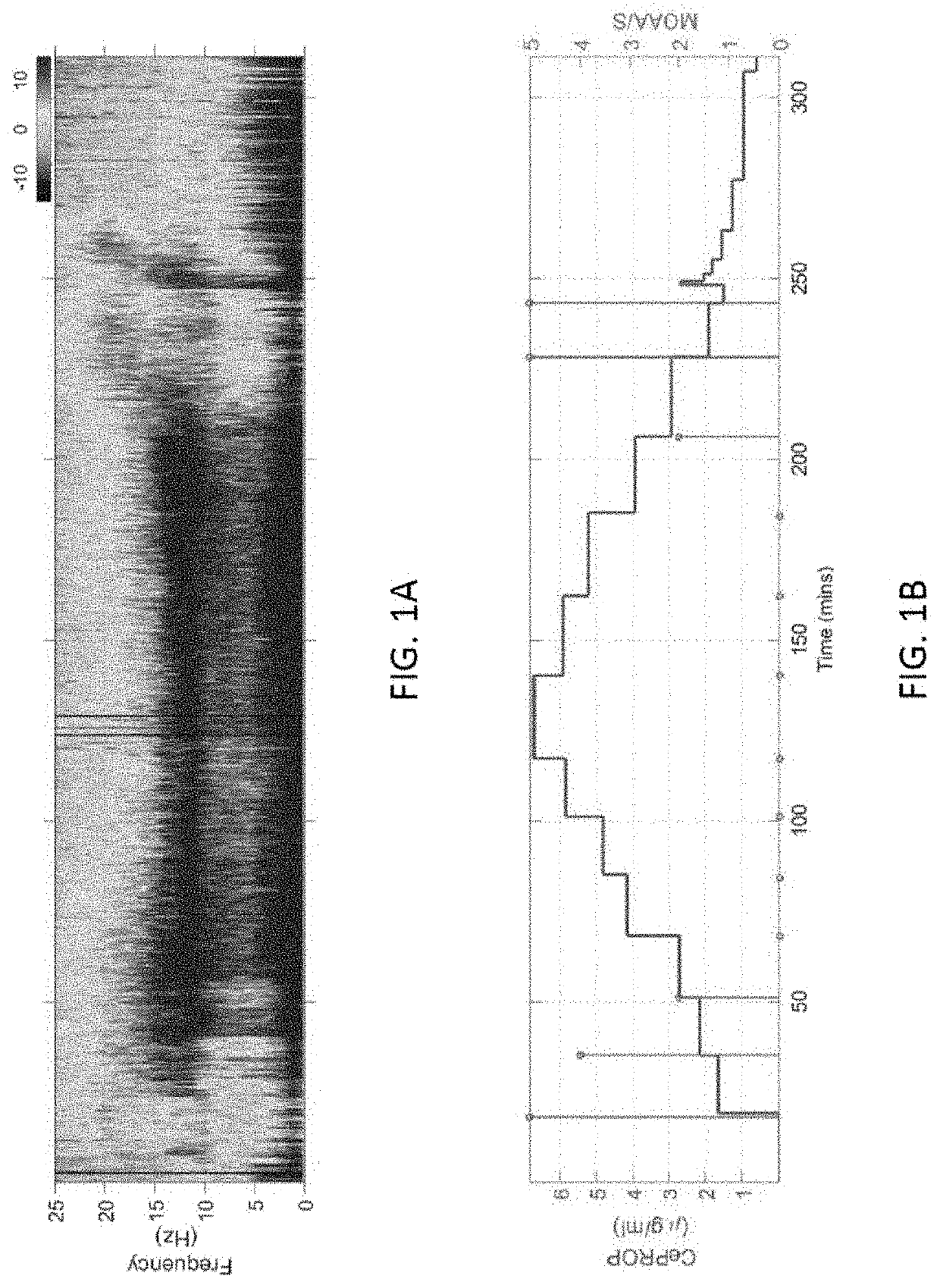
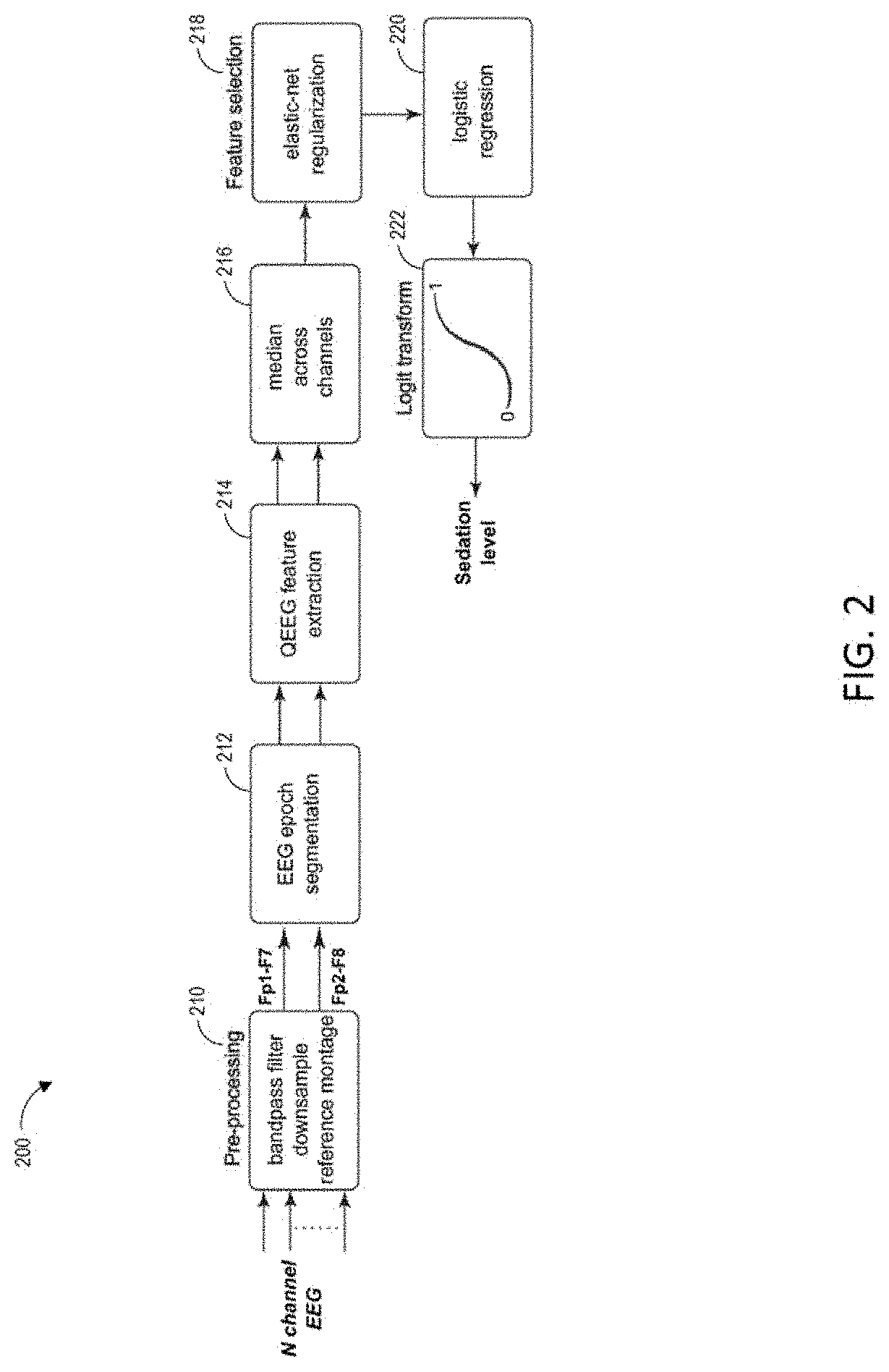
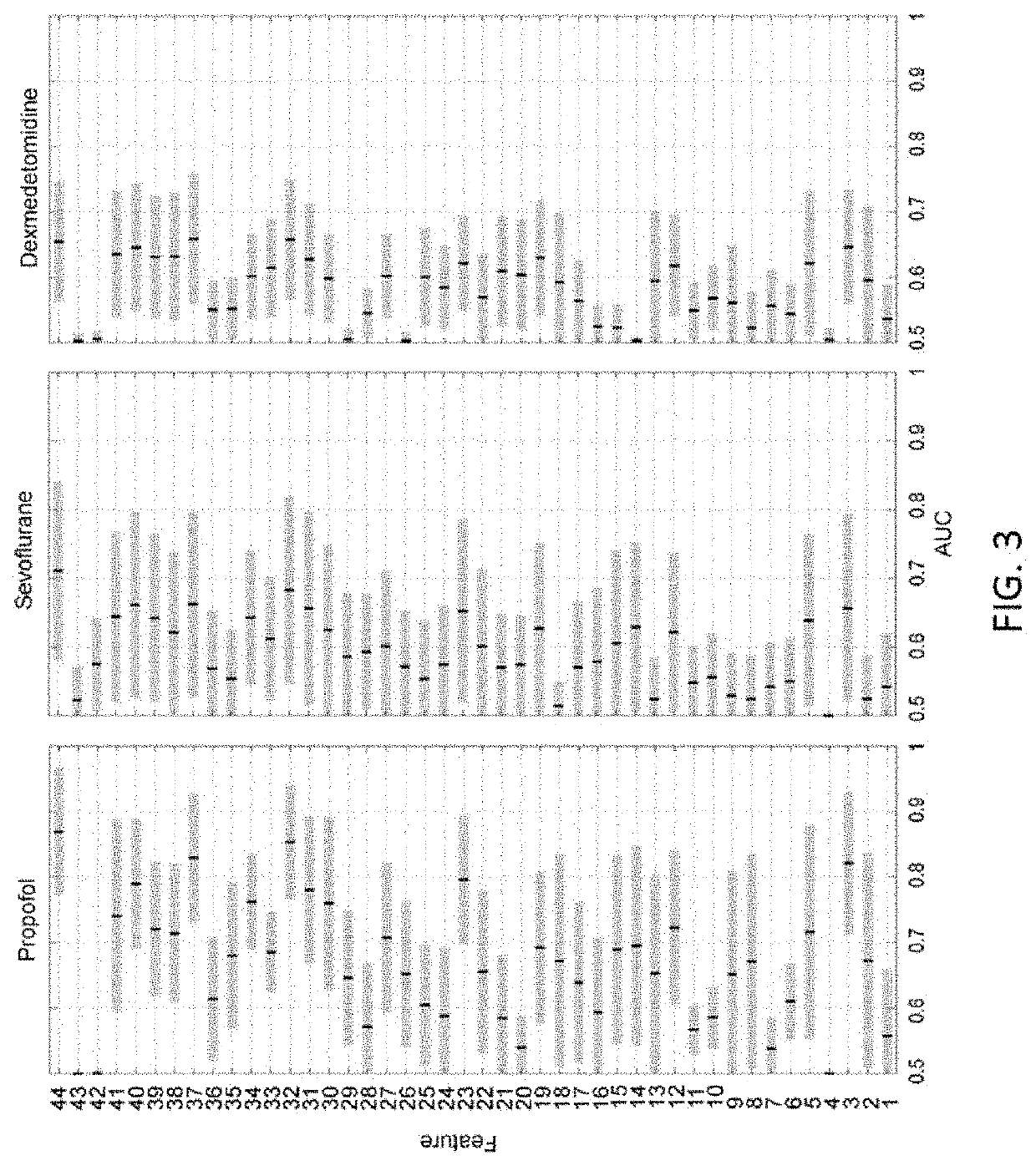
Combining multiple qeeg features to estimate drug-independent sedation level using machine learning
PendingUS20200253544A1Improve predictabilityImprove performanceElectroencephalographyMechanical/radiation/invasive therapiesSedative drugPhysical medicine and rehabilitation
The present disclosure describes systems and methods of estimating sedation level of a patient using machine learning. For example, the integration of multiple QEEG features into a single sedation level estimation system using machine learning could result in a significant improvement in the predictability of the levels of sedation, independent of the sedative drug used. The present disclosure advantageously allows for the incorporation of large numbers of QEEG features and machine learning into the next-generation monitors of sedation level. Different QEEG features may be selected for different sedation drugs, such as propofol, sevoflurane and dexmedetomidine groups. The sedation level estimation system can maintain a high performance for detecting MOAA / S, independent of the drug used.
Owner:MASIMO CORP
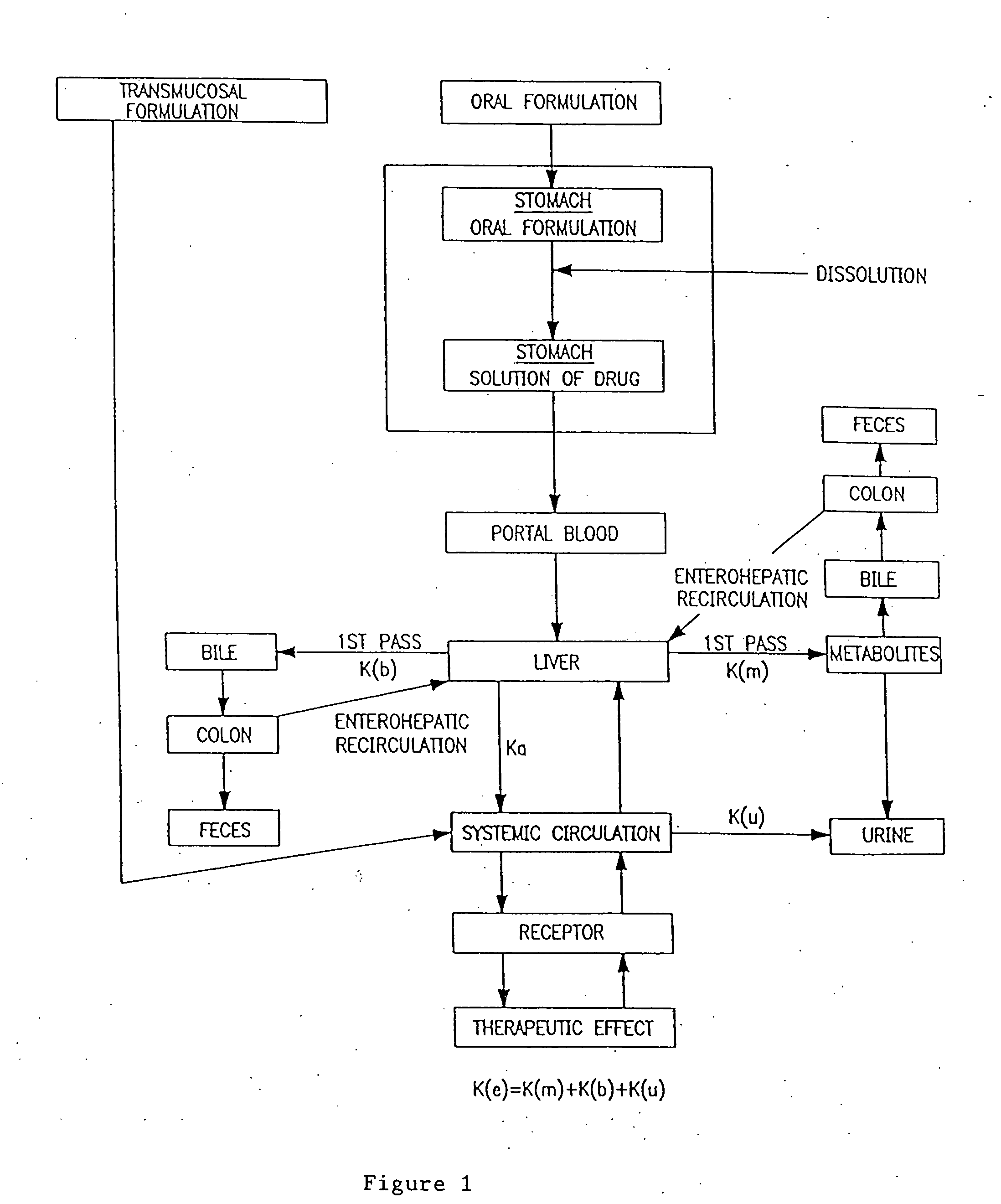
Buccal, polar and non-polar sprays containing propofol
InactiveUS20050002867A1Fast absorptionRapid onsetBiocideHydroxy compound active ingredientsAerosol spraySolvent
Buccal aerosol sprays using polar and / or non-polar solvents have now been developed which provide propofol for rapid absorption through the oral mucosa, resulting in fast onset of effect. The buccal polar compositions of the invention comprise formulation I: propofol, a polar solvent and an optional flavoring agent; formulation II: propofol, a polar solvent, a propellant, and an optionally flavoring agent; formulation III: propofol, a non-polar solvent, and an optional flavoring agent; formulation IV: propofol, a non-polar solvent, a propellant, and an optional flavoring agent; formulation V: propofol, a mixture of a polar solvent and a non-polar solvent, and an optional flavoring agent; and formulation VI: propofol, a mixture of a polar solvent and a non-polar solvent, a propellant, and an optional flavoring agent.
Owner:NOVADEL PHARMA
O/w-emulsions comprising semifluorinated alkanes
InactiveUS20140004197A1Improve antibacterial stabilityHigh drug loadingPowder deliveryBiocideAlkaneEmulsion
The invention provides liquid compositions in the form of physically stable emulsions comprising a semifluorinated alkane. The semifluorinated alkane is comprised in the dispersed phase, which may also include an active pharmaceutical ingredient. One of the preferred active ingredients is propofol. The compositions are optionally heat sterilisable and can be used for pharmaceutical or cosmetic product applications, and administered topically, intravenously, or via other routes.
Owner:NOVALIQ GMBH
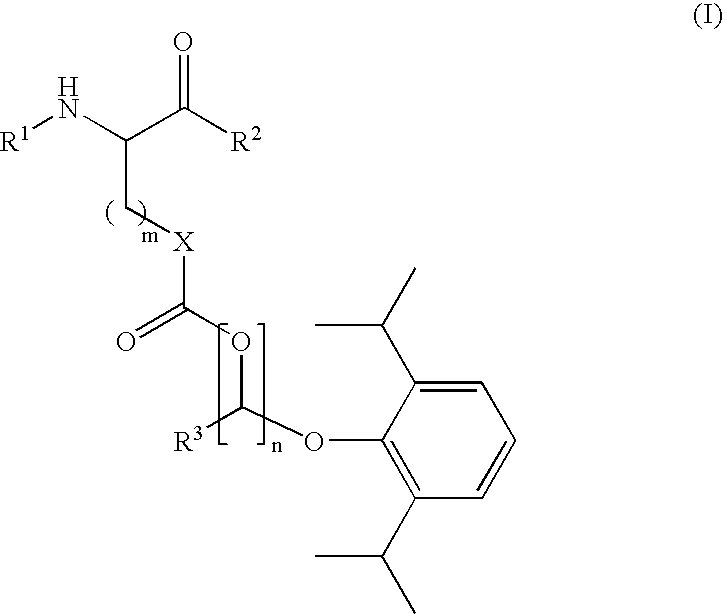
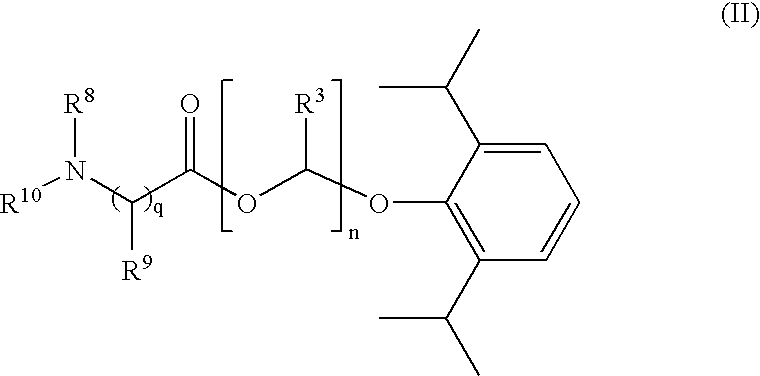
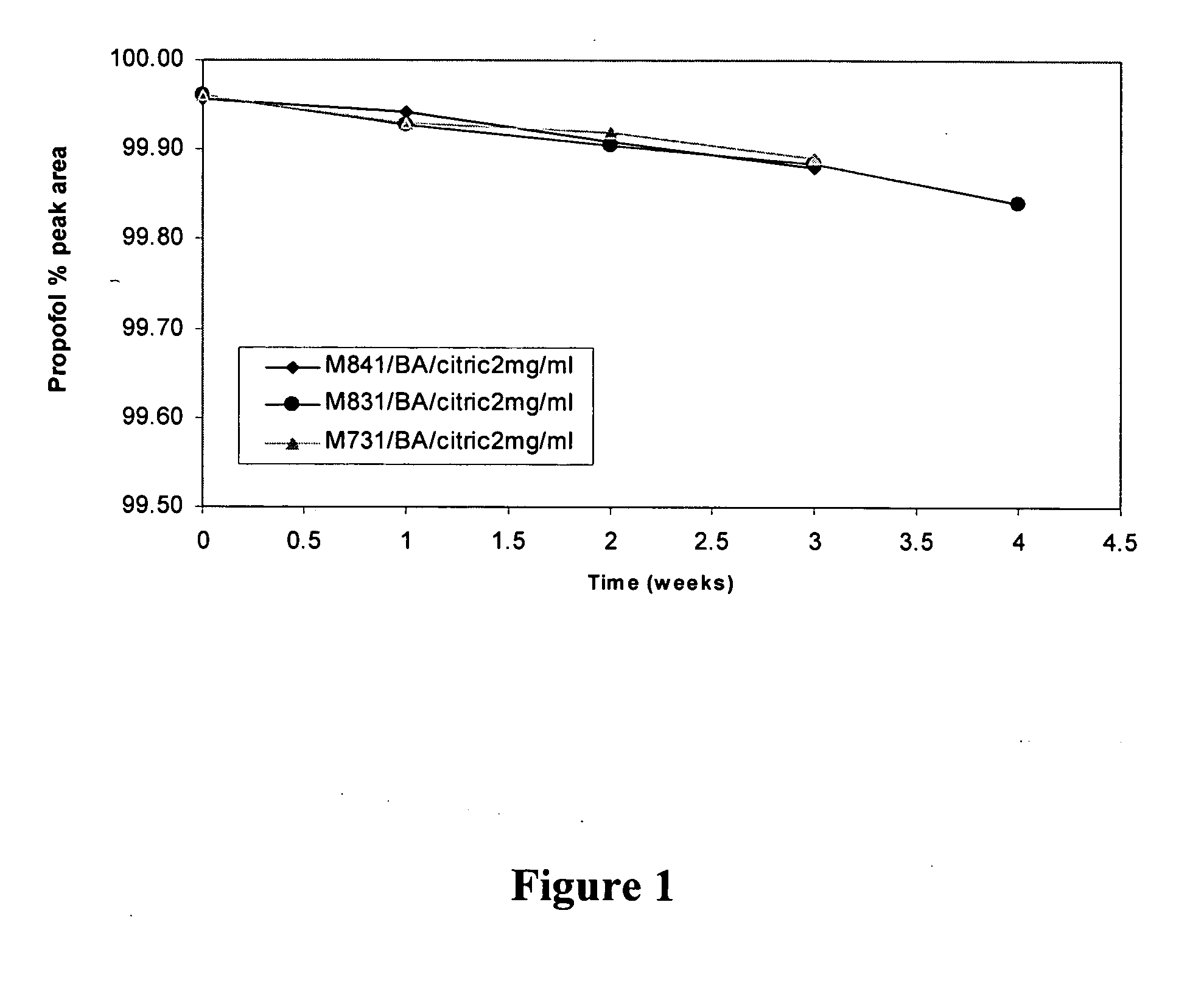
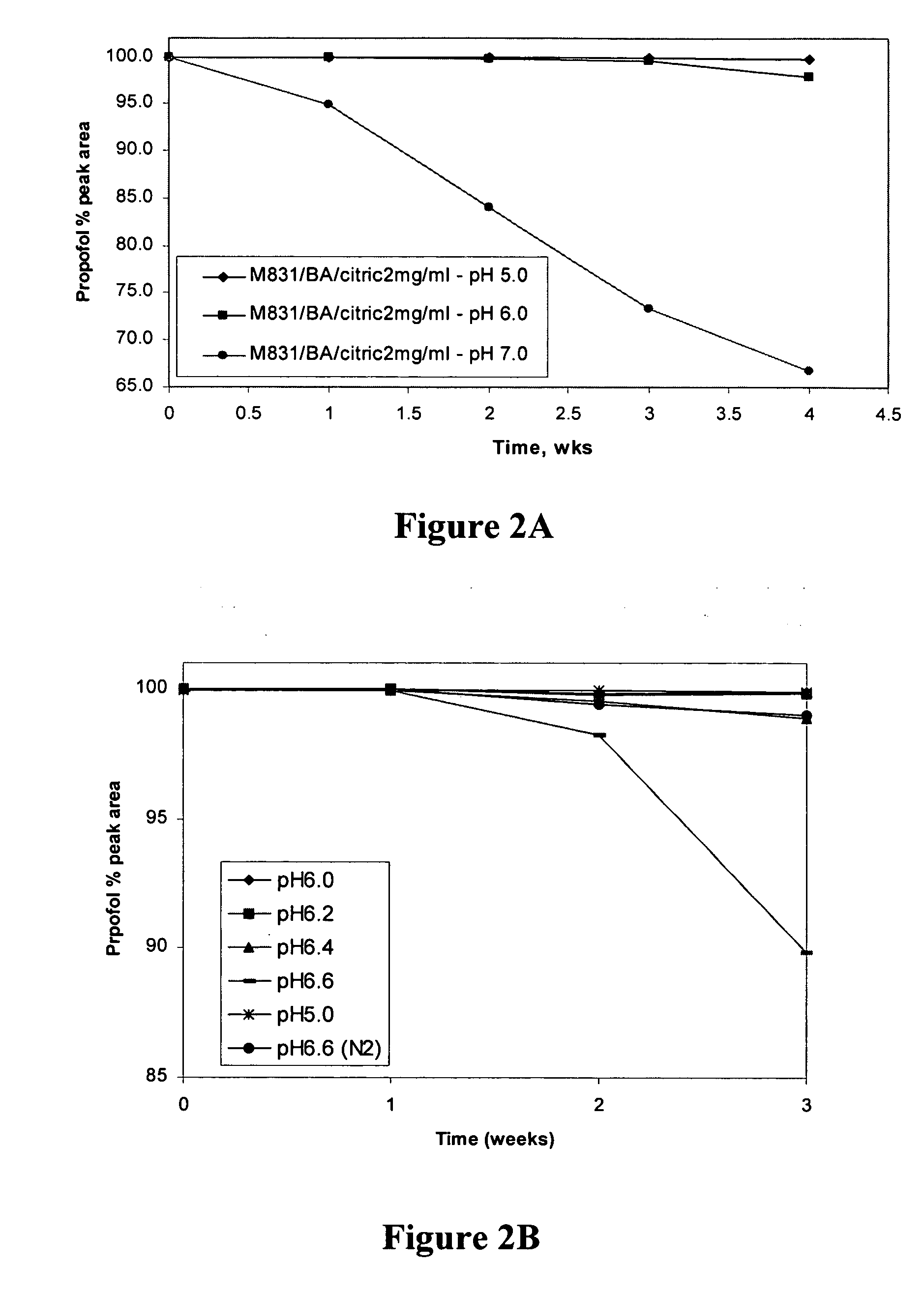
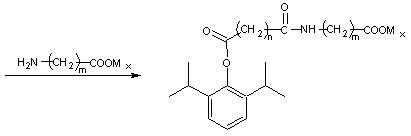
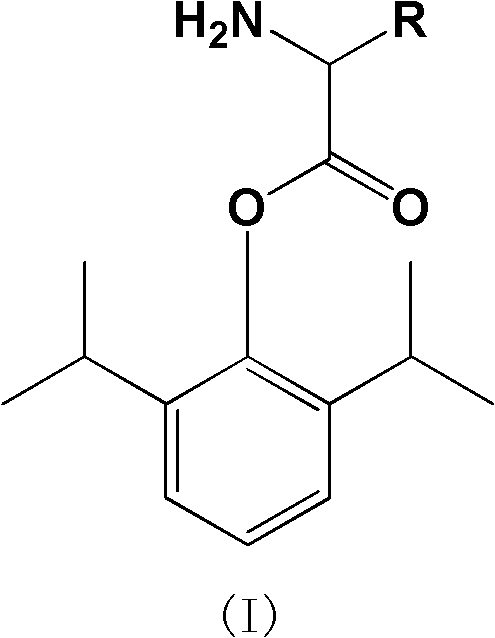
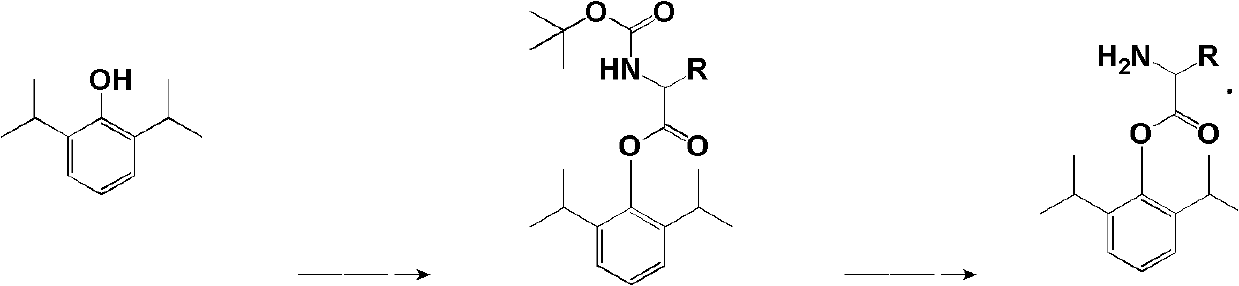
Amino acid derived prodrugs of propofol, compositions and uses thereof
The present invention provides propofol prodrugs, methods of making propofol prodrugs, pharmaceutical compositions of propofol prodrugs and methods of using propofol prodrugs and pharmaceutical compositions thereof to treat or prevent diseases or disorders such as migraine headache pain and post-chemotherapy or post-operative surgery nausea and vomiting.
Owner:XENOPORT
Aqueous pharmaceutical compositions of 2,6-diisopropylphenol (propofol) and their uses
InactiveUS20040265388A1Simple preparation processEasy to storePowder deliveryHydroxy compound active ingredientsPolyethylene glycolPropofol
The present invention provides aqueous pharmaceutical compositions containing a lipophilic therapeutic agent. In particular, the invention provides aqueous pharmaceutical compositions containing the compound 2,6-diisopropylphenol (propofol). Preferred compositions of the invention contain propofol in the presence of at least one block copolymer (for example, P188 or another poloxamer) and a polyethylene glycol (PEG). Compositions of the invention are preferably sterile or are readily sterilized (e.g., by autoclaving) and are suitable for parenteral administration to any animal, including humans. The compositions are also chemically and physically stable over a wide range of environmental conditions and for extended periods of time.
Owner:JANSSEN BIOTECH INC
Pharmaceutical composition comprising propofol
The invention provides novel pharmaceutical compositions comprising the active ingredient propofol. Preferably, propofol is dissolved in at least one semifluorinated alkane. The compositions, which are preferably liquid or gel-like, may optionally comprise further excipients. They may be used as fill material in capsules, as buccal or nasal sprays, or as aerosols for pulmonary administration. They are particularly useful for the transmucosal administration of propofol.
Owner:NOVALIQ GMBH
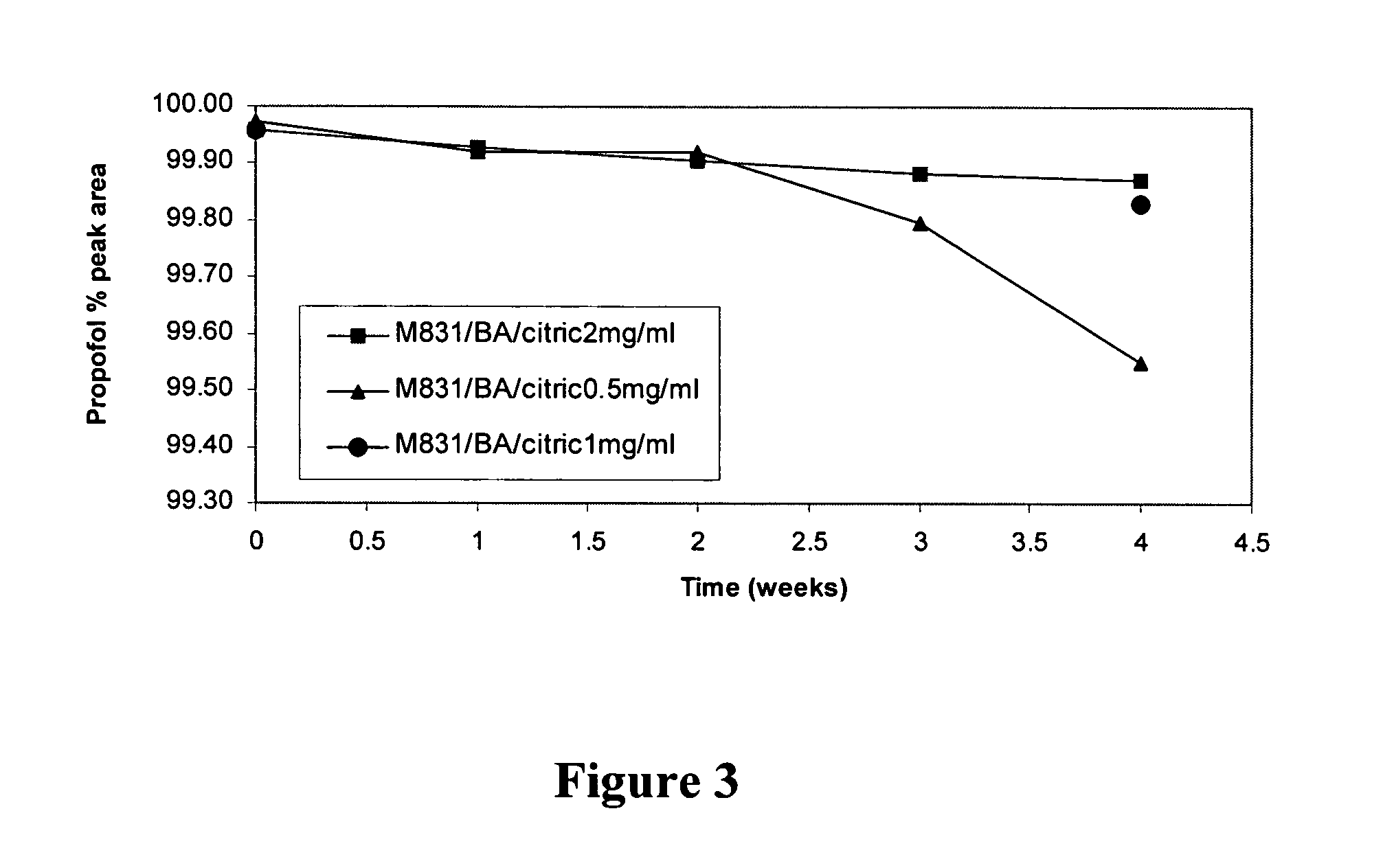
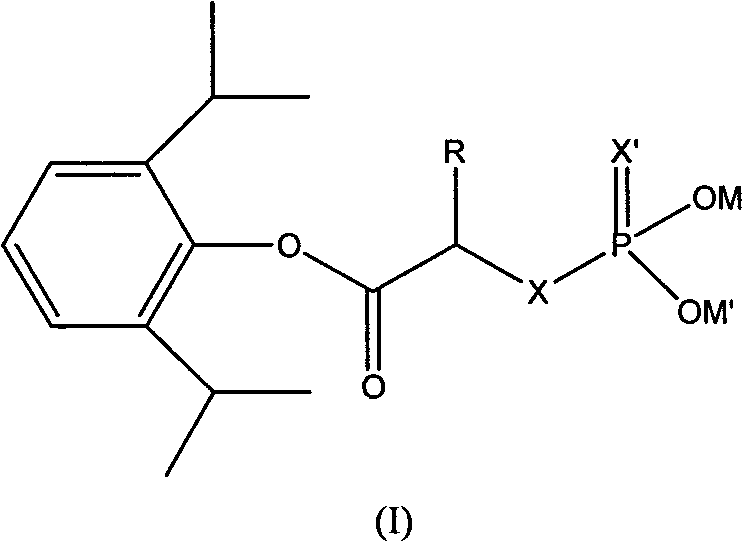
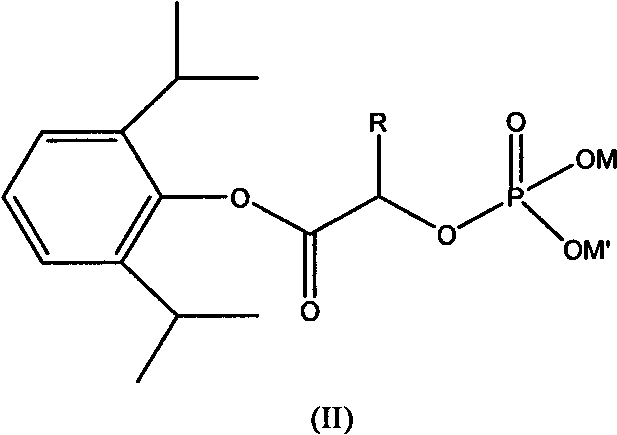
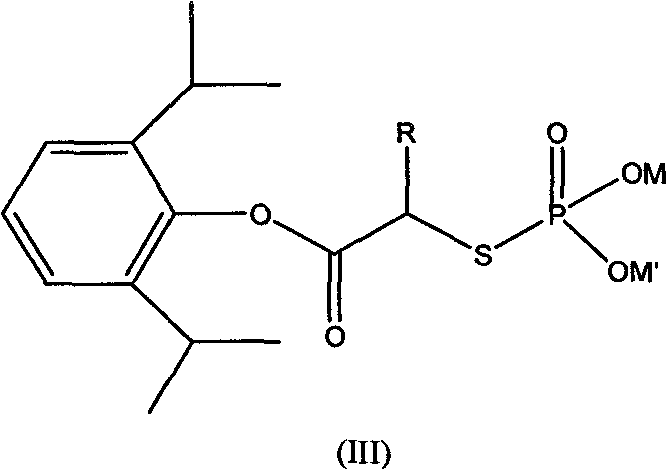
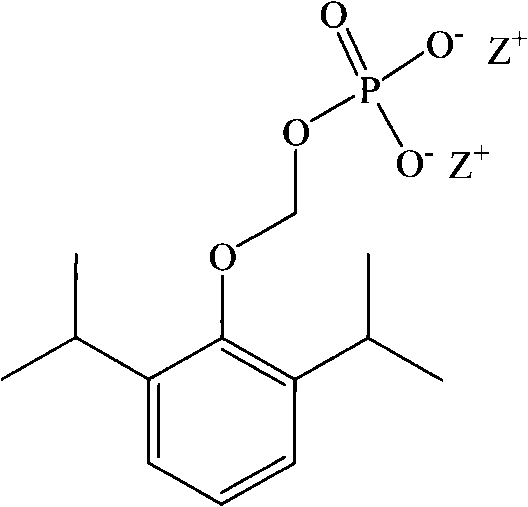
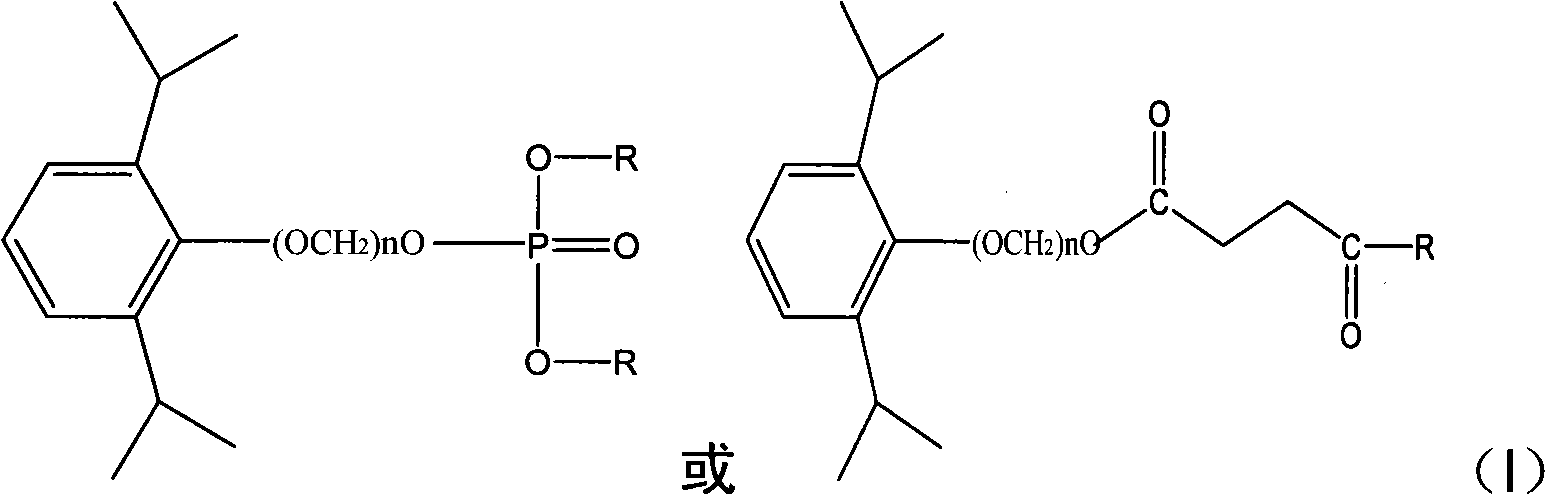
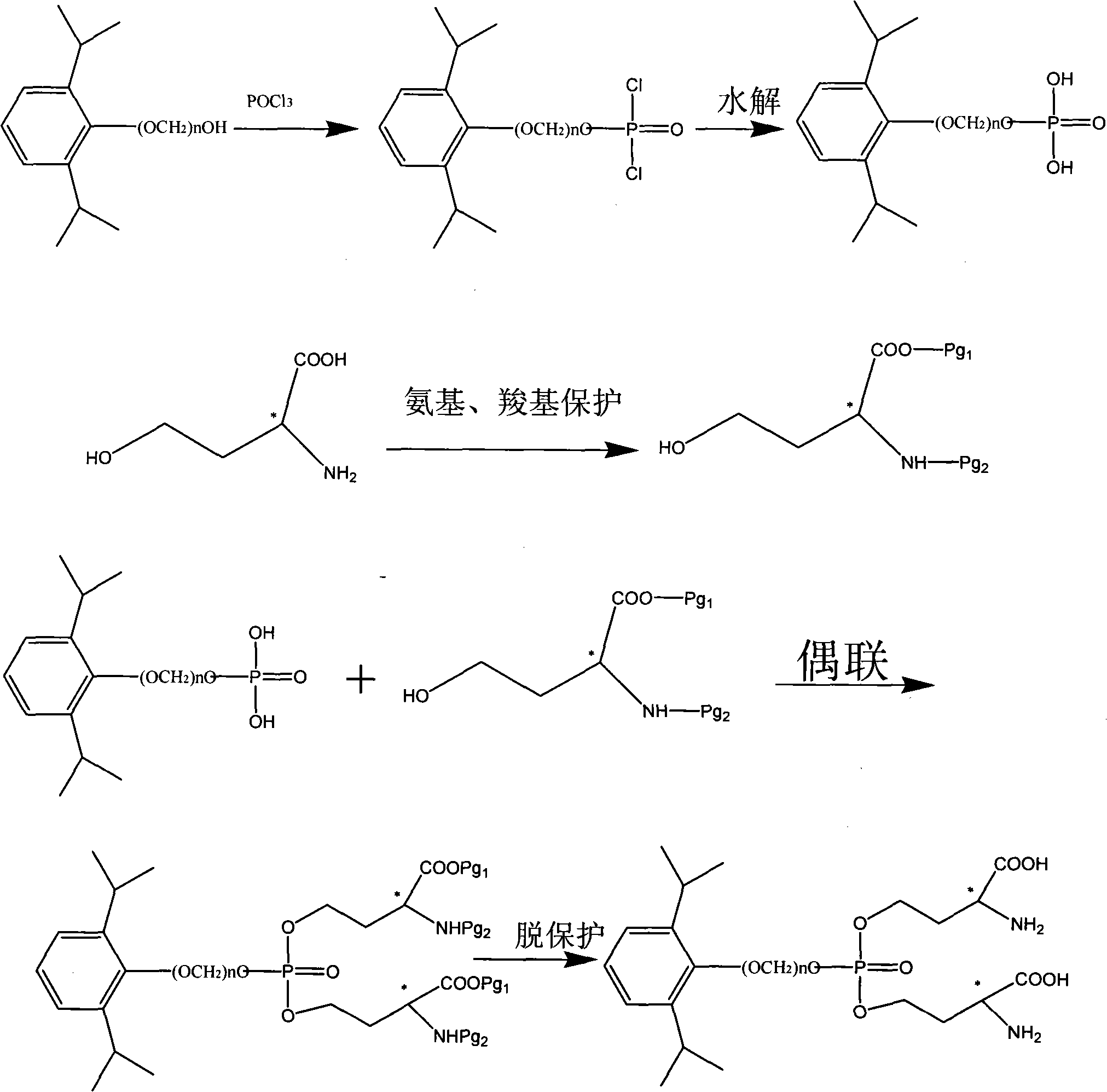
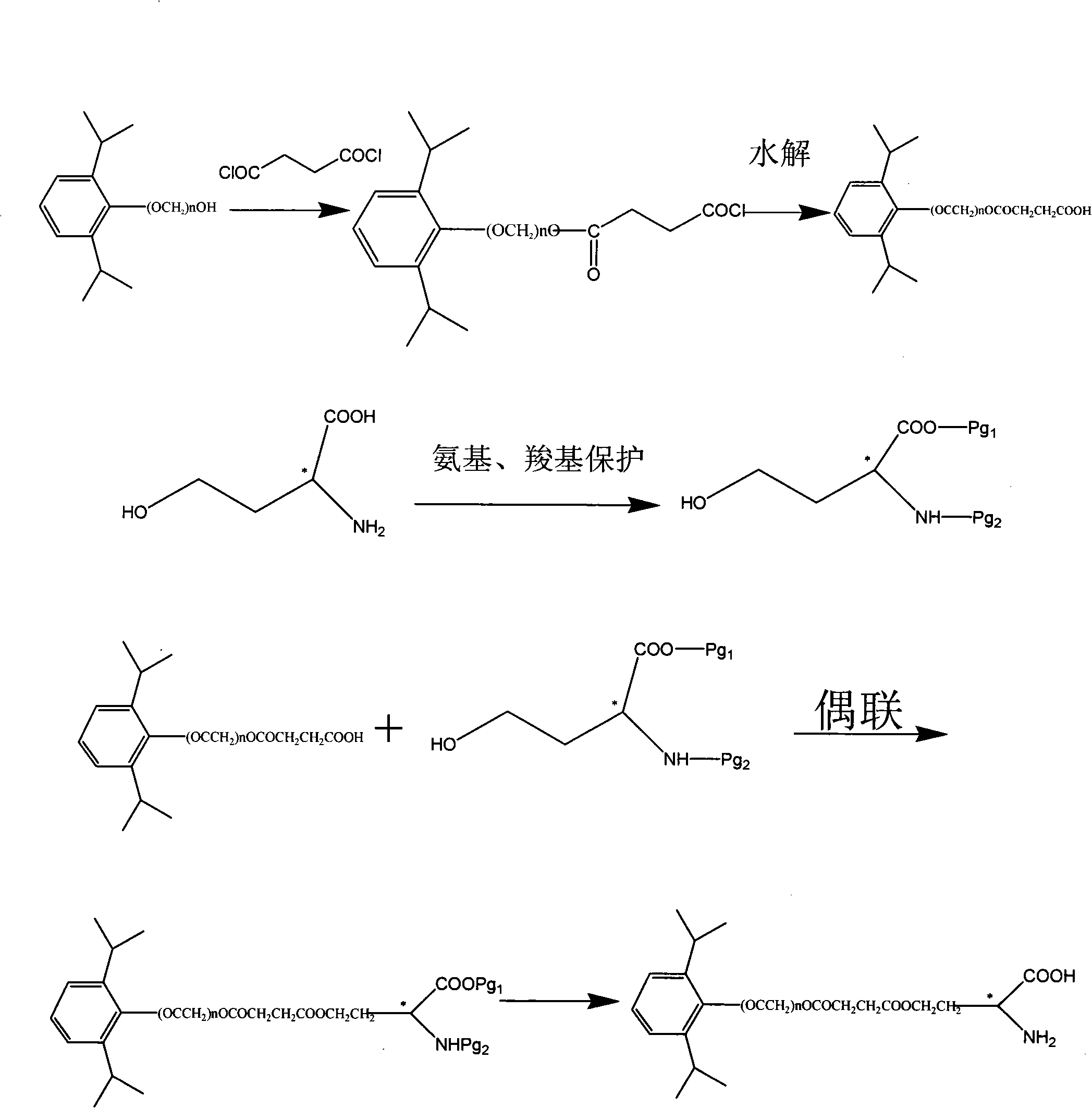
Phosphoryl carboxylic acid propofol ester derivative and preparation method thereof
ActiveCN101633671AImprove bioavailabilityGood water solubilityOrganic active ingredientsAnaestheticsO-Phosphoric AcidPhosphate
The invention relates to a phosphoryl carboxylic acid propofol ester derivative which has the general formula (I). The method comprises the following steps: propofol reacts with 2-halogenated carboxylic acid and a derivative thereof by alkali to obtain corresponding ester and then the product reacts with phosphoric acid or thiophosphoric acid and the derivative thereof by dissolvent to obtain a water-soluble product or the propofol reacts with a 2-halogenated carboxylic acid phosphate ester derivative by the alkali to obtain the corresponding ester and then the ester is catalyzed, hydrogenated and salified to obtain the water-soluble product (I). The preparation method has mild reaction condition, high yield, simple operation and industrialized prospect, and a prepared oral preparation has the characteristics of high bioavailability, rapid absorption, high stability, and the like; and auxiliary materials with safety defects, such as a surface active agent, and the like can not be added into the prepared injection, thereby improving the stability of the preparation, reducing or removing injection pain, increasing the compliance of patients, overcoming the defects of propofol emulsion and having the advantage of obvious effect. The invention has the structural general formula (I).
Owner:HANGZHOU ADAMERCK PHARMLABS INC
Method for monitoring propofol narcotic in on-line manner
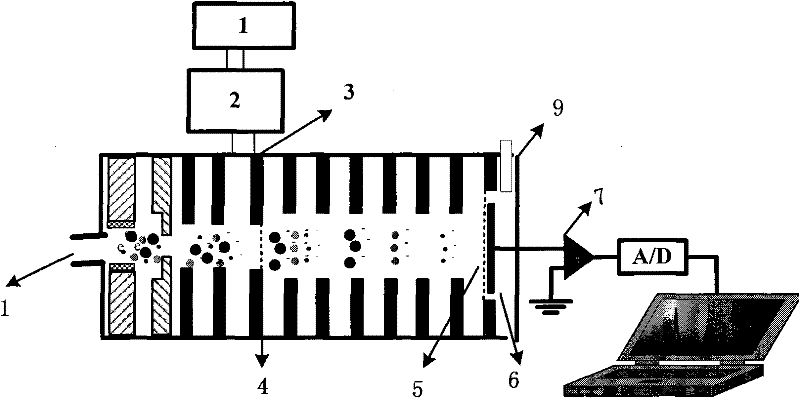
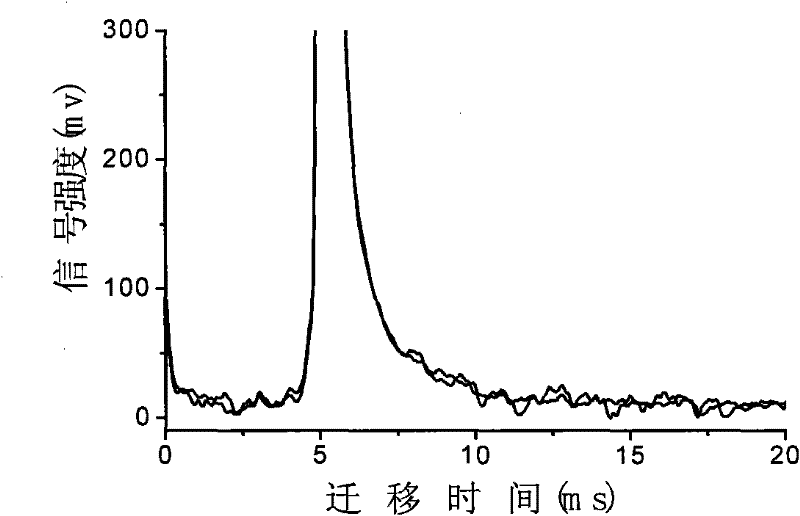
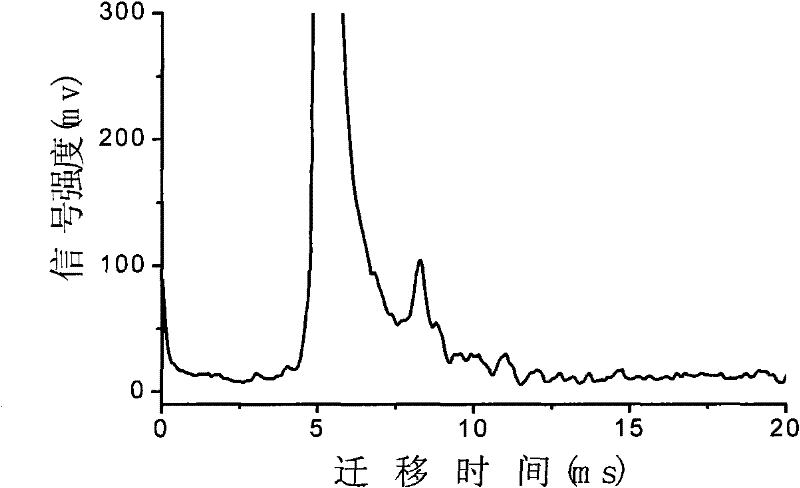
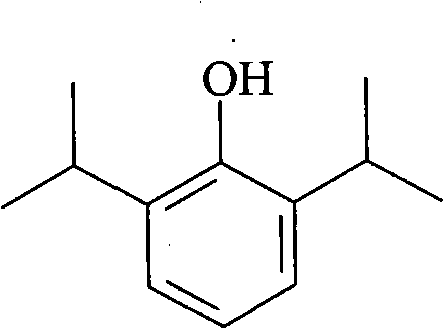
InactiveCN102455319AReduce volumeShort analysis timePreparing sample for investigationMaterial analysis by electric/magnetic meansIon-mobility spectrometryAnesthetic
The invention discloses a method for monitoring traced propofol narcotic rapidly and sensitively. In the method, an ion mobility spectrometry technology is taken as a basic detection technology, and an anion mode is adopted to establish a method for monitoring traced propofol narcotic. The method comprises the following steps: feeding a gas sample containing propofol into an ion mobility spectrometry tester for analysis to obtain a detecting signal, and then carrying out qualitation and quantitation. According to the invention, the measurement of a narcotic sample can be detected in real time, and the limit of detection of the propofol narcotic can reach 0.5ppb. The detecting method is simple, convenient, rapid and efficient and is good in reliability.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Formulations for anaesthetic use
InactiveUS20050004234A1Risk can be causedRisk of causing anaphylactoid reactions in susceptible individuals isBiocideHydroxy compound active ingredientsLipid formationSedation
A formulation for anaesthetic use is described. The formulation contains propofol, and may be used to induce and / or maintain anaesthesia or sedation in a vertebrae. The formulation additionally contains a solvent or a combination of solvents and is suitable for mixing with commonly used infusion fluids prior to injection in to a patient. The formulation may be terminally sterilised using moist heat in order to assure sterility, and contains no lipid, thereby avoiding complications associated with administration over prolonged periods of time, or to patients with disorders of fat metabolism.
Owner:PARNELL TECH
Formula and preparation method of novel propofol fat emulsion preparation causing no pain and low injection stimulation
ActiveCN102085185ADissolve effectively and fullyHydroxy compound active ingredientsAnaestheticsGlycerolSugar
The invention discloses a formula and preparation method of a novel propofol fat emulsion preparation which causes no pain or can be used for obviously lowering injection stimulation and pain. The novel preparation comprises the main constituents of propofol, an emulsifier, refined soybean oil or other refined oil as an oil-soluble diluent, oleic acid or oleate with the effect of assisting emulsification, vitamin E or derivatives thereof with an antioxidation effect, an ionic complexing agent, and glycerol or micro molecular sugar as an isoosmotic adjusting agent.
Owner:XIAN LIBANG PHARMA
Propofol injection and its preparing method
InactiveCN101045042AImprove securityLess irritatingHydroxy compound active ingredientsPharmaceutical delivery mechanismDisodium EdetateHigh pressure
Owner:SHANGHAI INST OF PHARMA IND
New precursor medicinal preparation
ActiveCN101716149AInhibit devitrificationPrevent precipitationPowder deliveryNervous disorderSolubilityFreeze-drying
The invention discloses a freeze-drying preparation of a water-solubility propofol precursor compound and a preparation method thereof. The freeze-drying preparation of the propofol precursor compound, prepared in the method of the invention, has the advantages of favorable water solubility, stability and safety.
Owner:YICHANG HUMANWELL PHARMA
PH sensitive prodrugs of 2,6-Diisopropylphenol
The present invention is directed to water-soluble derivatives of 2,6-diisopropylphenol (Propofol). The compounds act as prodrugs of 2,6-diisopropylphenol and metabolize rapidly to Propofol thereby providing an alternative to the water-insoluble 2,6-diisopropylphenol. Pharmaceutical compositions comprising these compounds, methods of induction and maintenance of anesthesia or sedation as well as methods of treating neurodegenerative diseases utilizing pharmaceutical compositions comprising these compounds and methods of preparing them are also disclosed.
Owner:AUSPEX PHARMA INC
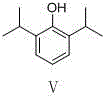
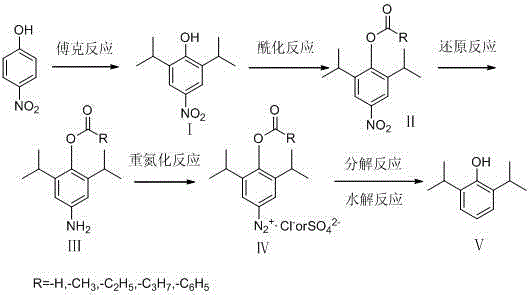
Preparation method of propofol
The invention provides a preparation method of propofol, which includes following steps: (1) performing Friedel-crafts reaction to p-nitrophenol and isopropanol or 2-halogenated propane under catalysis of an acid to prepare a compound I; (2) performing acylation protection to the compound I to prepare a compound II; (3) performing a reduction reaction to the compound II to prepare a compound III; (4) performing a diazo-reaction to the compound III to prepare a compound IV; and (5) under a weak reducing agent condition, performing a decomposition reaction to the compound IV and meanwhile carrying out hydrolysis under an alkaline condition to obtain the propofol V. The raw materials of the preparation method are easy to obtain. The preparation method is simple in process and is high in yield.
Owner:LIAONING YAOLIAN PHARMA
Propofol freeze-dried emulsion and its preparing method
InactiveCN101006992ANo significant change in particle sizeImprove stabilityHydroxy compound active ingredientsAnaestheticsEmulsionFreeze-drying
The invention relates to a medicinal composition containing propofol and process for preparation, wherein each 1kg of the freeze-dried product comprises propofol 1g, oil for injection 0-20g, emulsifying agent 0.1-10g, auxiliary emulsifying agent 0.001-5g, other stabilizing agent 0-2g, freeze-drying protective agent 1-40g, and right amount of pH modifier. The invention can achieve better constancy.
Owner:姚瑶 +1
Clear aqueous composition comprising propofol and hydroxypropyl-beta-cyclodextrin
ActiveUS7138387B2No added fat loadImprove stabilityBiocideHydroxy compound active ingredients2 hydroxypropyl β cyclodextrinPhenol
Sterile pharmaceutical stable autoclaved clear aqueous compositions of propofol (2,6-Diisopropyl phenol) suitable for parenteral administration are described. The compositions essentially consist of a complex of propofol with 2-hydroxypropyl-β-cyclodextrin in a weight ratio of 1:30–1:60. This complex of propofol with 2-hydroxypropyl-β-cyclodextrin produces a clear aqueous composition that is stable to autoclaving. The composition is effective as an anaesthetic agent. The process of making these synergistic compositions has been described.
Owner:BHARAT SERUMS & VACCINES
Intravenous Propofol emulsion compositions having preservative efficacy
The invention discloses a stable intravenous Propofol oil-in-water emulsion composition having mixed preservatives of low toxicity that is capable of withstanding accidental contamination of bacteria and fungi. The preservative system employed comprising of monoglyceryl ester of lauric acid (Monolaurin) and a member selected from (a) capric acid and / or its soluble alkaline salts or its monoglyceryl ester (Monocaprin); (b) edetate; and (c) capric acid and / or its soluble alkaline salts or its monoglyceryl ester (Monocaprin) and edetate.
Owner:BHARAT SERUMS & VACCINES
Propofol-containing fat emulsion preparation
InactiveCN1909893ALittle side effectsReduced stabilityNervous disorderHydroxy compound active ingredientsAnesthetic AgentCyclodextrin Derivatives
Owner:OTSUKA PHARM FAB INC
Double-effect anesthetic and preparation method and application thereof
ActiveCN105753701AReduce the incidence of injection painReduce severityOrganic active ingredientsPreparation from carboxylic acid halidesPropofol InjectionSeverity/Intensity
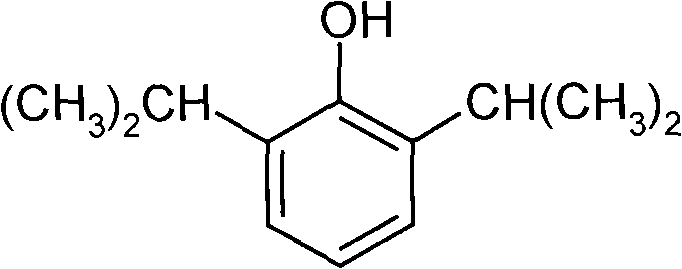
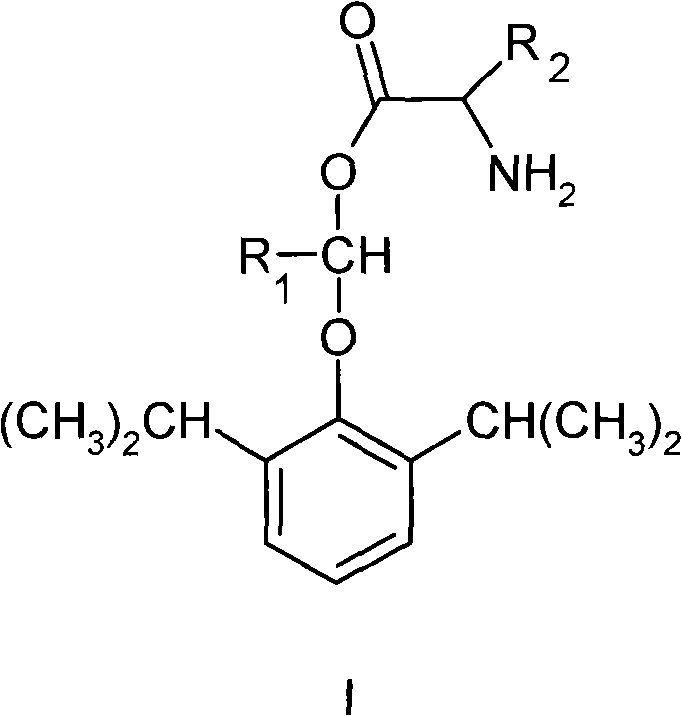
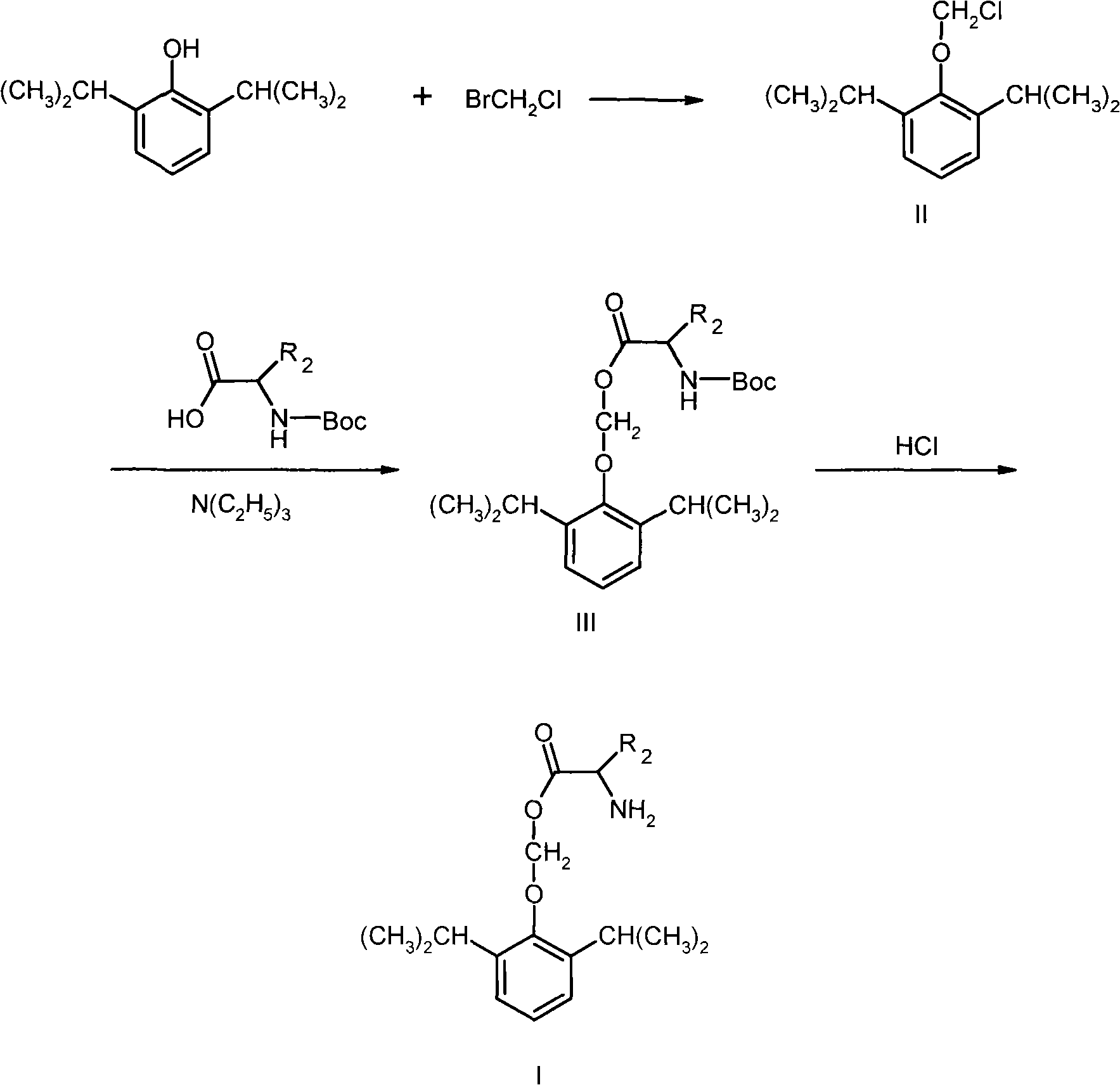
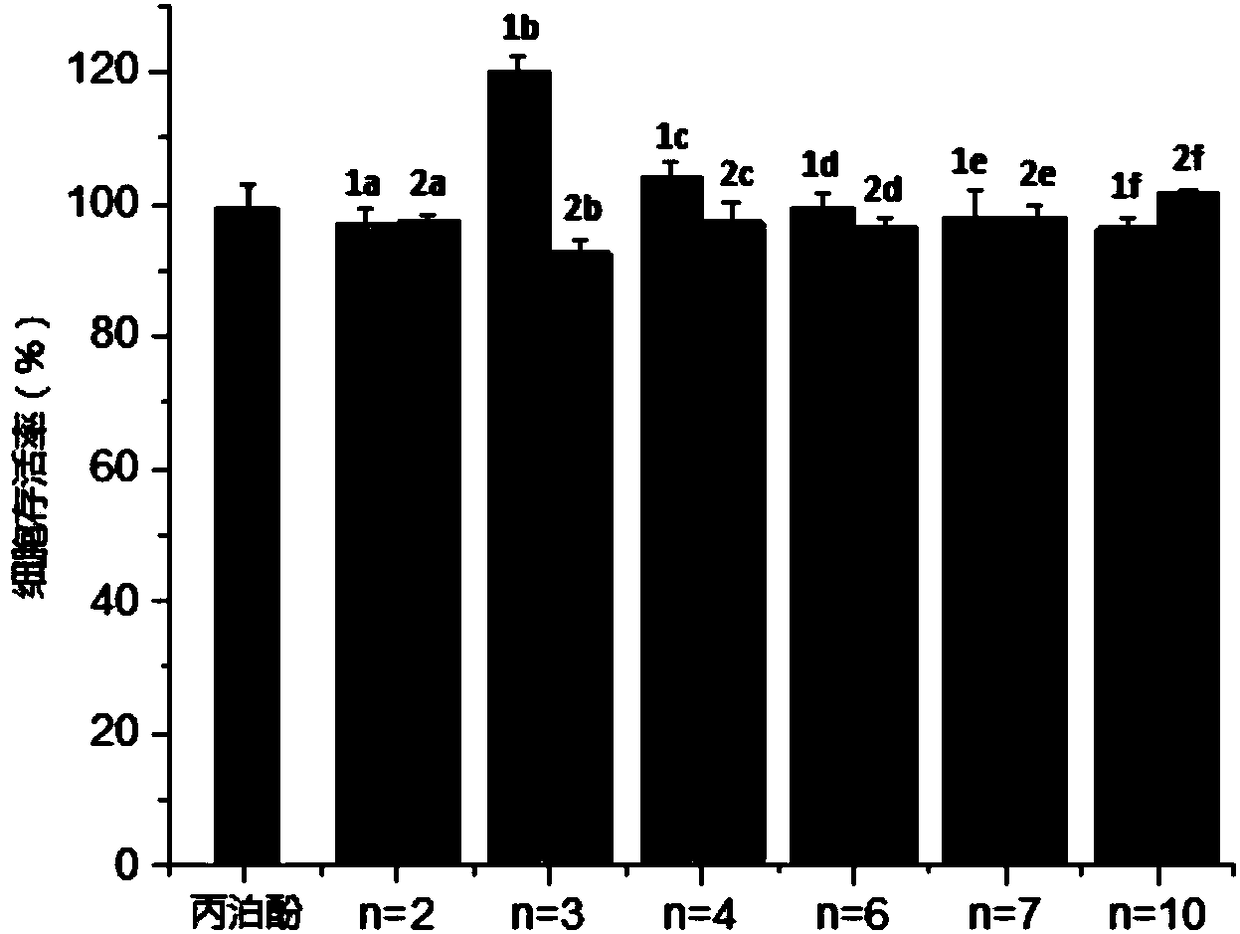
The invention belongs to the technical field of medicine and particularly relates to a double-effect anesthetic and a preparation method and application thereof.The invention discloses a compound shown in the general formula (I).The compound can generate analgesic flurbiprofen and anesthetic propofol after hydrolysis, the analgesic and the tranquilizer are combined, dosage frequency of anesthetists is reduced, the anesthetic process is quicker, the occurrence rate and the severity of propofol injection pain can be remarkably reduced, adverse reactions of single drug are reduced, and the anesthetic has the advantages that the anesthetic takes effects quick, lasting time is short, anesthetic efficiency is high, controllability in operation is high, and patients recover quickly.Please see the structural formula in the description, wherein R refers to O or a radical group in the description, and n is 2-4.
Owner:GUANGDONG JIABO PHARM CO LTD
Propofol flexible nano-liposome patch and application thereof
ActiveCN106214668AQuick effectGood effectNervous disorderHydroxy compound active ingredientsLiposomeColloidal Solution
The invention discloses a propofol flexible nano-liposome patch which can promote sleeping and improve the sleeping quality. The patch is composed of a propofol flexible nano-liposome solution and a substrate layer. The propofol flexible nano-liposome solution is mainly composed of propofol, phospholipids and a flexible agent, wherein the grain size of the solution is tens to hundreds nanometers, and a colloidal solution exists on the appearance. The patch has the advantages of high deformation, high hydrophilia, efficient permeability and high stability.
Owner:XIAN LIBANG PHARMA
Propofol ester derivative containing amino carboxylic acid amide structure, its preparation method and its purpose
ActiveCN102382005AOvercome the disadvantage of poor water solubilitySmall toxicityOrganic active ingredientsNervous disorderAlkaline earth metalSide effect
The invention relates to a propofol ester derivative containing an amino carboxylic acid amide structure, its preparation method and its purpose. The derivative has a following formula (I), in the formula, M is H, NH4 or pharmaceutically acceptable alkali metal element or alkali earth metal element, n=1-3, m=1-7, x=1 / 2-1. 2,6-diisopropyl phenol (II) is taken as a raw material, reacted with diacid or the corresponding anhydride to obtain a 2,6-diisopropyl phenol monoester intermediate, and continuously reacted with amino carboxylic acid to obtain the corresponding product (I). The propofol ester derivative can be used for preparing injection type central inhibition medicaments for generating effects of calmness, hypnosis and / or anaesthesia on animals and human, and is capable of improving the water-solubility of propofol, reducing the toxic and side effects of original drug and analogous prodrug, increasing the stability of profrug in vitro and increasing the application scope. The preparation method is simple which has industrial application value.
Owner:SICHUAN UNIV
Water-soluble amino acid ester derivative of propofol and application thereof
The invention relates to a water-soluble amino acid ester derivative of propofol and application thereof. Amino acid ester of the propofol has a structure with a formula (I), wherein R is shown in the description. The invention also relates a preparation method for a compound with the formula (I), a medicinal salt thereof, a compound-containing medicinal composition and application of the compound serving as narcotic medicines.
Owner:CHENGDU YIPING MEDICAL SCI & TECH
Water-soluble amino-acid ester derivative of propofol
The invention relates to a water-soluble amino-acid ester derivative of propofol shown in a formula (I), nontoxic pharmaceutically-acceptable salts thereof, a pharmaceutical composition which contains the compound and is used as an active ingredient, and the application of the compound and the pharmaceutical composition as narcotic drugs, wherein R1 is an alkyl of H or C1-C3, and R2 is a lateral chain of L-amino acid of hydrogen, methyl, isopropyl, isobutyl, 2-methyl-propyl or benzyl, and the like.
Owner:BEIJING MEIBEITA DRUG RES
Preparation and application of monodisperse polyethylene glycol monomethyl ether modified propofol prodrug
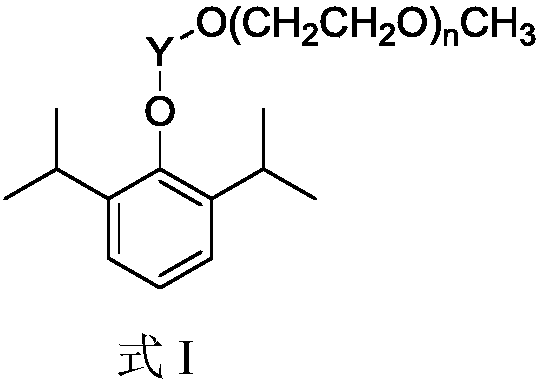
Belonging to the field of organic chemistry and pharmaceutical chemicals, the invention discloses preparation and application of a monodisperse polyethylene glycol monomethyl ether modified propofol prodrug with a novel structure. Monodisperse polyethylene glycol monomethyl ether and propofol can be connected by an in-vivo degradable chemical bond to prepare a series of carbonic ester type and acetic ester type prodrugs respectively. By adjusting the length of the monodisperse polyethylene glycol monomethyl ether chain, the method can synthesize corresponding water-soluble propofol prodrugs, the longer the monodisperse polyethylene glycol monomethyl ether chain is, the better the water solubility is, along with the improvement of the water solubility, the prodrug can be made into aqueous solution preparations so as to avoid the defects of fat emulsion in propofol fat emulsions. The method provided by the invention has the advantages of simple reaction, mild conditions and low cost, andis convenient for industrial production.
Owner:WUHAN UNIV
Delivery systems for propofol
ActiveUS10568834B2Improve stabilityReduce the risk of contaminationHydroxy compound active ingredientsAntipyreticMicroemulsionMedicinal chemistry
Owner:YISSUM RES DEV CO OF THE HEBREWUNIVERSITY OF JERUSALEM LTD
A propofol composition used for injection, a preparing method thereof and uses of the propofol composition
InactiveCN104288130ANo delaminationImprove liquidityNervous disorderHydroxy compound active ingredientsFiltration membraneCLARITY
The invention belongs to the field of pharmacology and pharmaceutics, and relates to a propofol composition used for injection, a preparing method thereof and uses of the propofol composition. A product of the propofol composition is good in fluidity and free of wall hanging, has a single-phase, transparent, clear and bright appearance, can be subjected to clarity detection, and is free of preparation layering phenomena after the product is repeatedly frozen and thawed. In a process of forming a propofol fat emulsion system when the product encounters water, homogenization treatment is not needed, and spontaneous emulsification can occur only by slight oscillation. During clinical using, the product can spontaneously emulsify to form a fat emulsion system meeting injection requirements after the product is diluted with normal saline or a glucose solution, and other aqueous solutions and is slightly oscillated. A preparation process is simple. Only a common physical stirring step is needed, and a homogenizing step or a water removing step is not needed. A prepared propofol fat emulsion concentrate liquid can be sterilized by passing through a microporous filtration membrane having a size of 0.22 [mu]m. After emulsification, the content of free propofol is low, and the phenomenon of pain during administration caused by existence of the free propofol is reduced. The propofol composition, the preparing method and uses have advantages of simple preparation process, low production cost, and easy transportation and storage, and have a good application prospect.
Owner:天津迈迪瑞康生物医药科技有限公司
Application of small dose of propofol in preparing products for preventing and curing post-traumatic stress disorder (PTSD)
InactiveCN106109449ASignificant anti-PTSD behavioral effectsPrevent, alleviate and/or treat stress disordersNervous disorderHydroxy compound active ingredientsAcute Stress DisorderIrritability
The invention discloses application of a small dose of propofol in preparing products for preventing and curing post-traumatic stress disorder (PTSD). Stress disorder and its related diseases include acute stress disorder (ASD) and PTSD. According to the test of injecting a small dose of propofol in the abdominal cavity of a PTSD pattern animal, the small dose of propofol has function of relieving fear, anxiety and learning and memory impairment of the PTSD pattern animal, and can decrease expressions of inducible nitric oxide synthase (iNOS) and neurons nitric oxide synthase (nNOS) which are caused by strong stress, increase expression of brain derived neurotrophic factor (BDNF) which plays an important role in neuroplasticity regulation and reduce release of nitric oxide (NO) of glioma cell of the pattern animal greatly. Accordingly, propofol with the advantages of quick action, quick metabolic clearance and no residues has good application prospect.
Owner:THE 309TH HOSPITAL OF CHINESE PEOPLES LIBERATION ARMY
Carbonic acid diester water-soluble derivant of amino acid and propofol, and application of carbonic acid diester water-soluble derivant of amino acid and propofol
ActiveCN102617380ANo side effectsGood water solubilityOrganic active ingredientsNervous disorderWater solubleAmino acid
The invention relates to a carbonic acid diester water-soluble derivant of amino acid and propofol, and application of the carbonic acid diester water-soluble derivant of amino acid and propofol. The amino acid carbonic acid diester of propofol has the structure which is shown as a formula (1), wherein R is shown in the specifications; and the invention also discloses a preparation method for the compound with the formula (I), pharmaceutical salt of the compound, a medical composition of the compound and the pharmaceutical salt of the compound, and application of the compound used as a dope.
Owner:CHENGDU YIPING MEDICAL SCI & TECH
Propofol derivative for therapy
InactiveCN101781329AImprove solubilityPharmacologically activeOrganic active ingredientsAnaestheticsMedicineHydroxy compound
The invention provides a propofol derivative as well as a preparation method and purpose thereof. The propofol derivative is obtained by chemically modifying the hydroxyl group in the structure of propofol. The related propofol derivative can be used in pharmaceutical composition and can be used for producing medicaments for anaesthesia.
Owner:HC SYNTHETIC PHARMA CO LTD
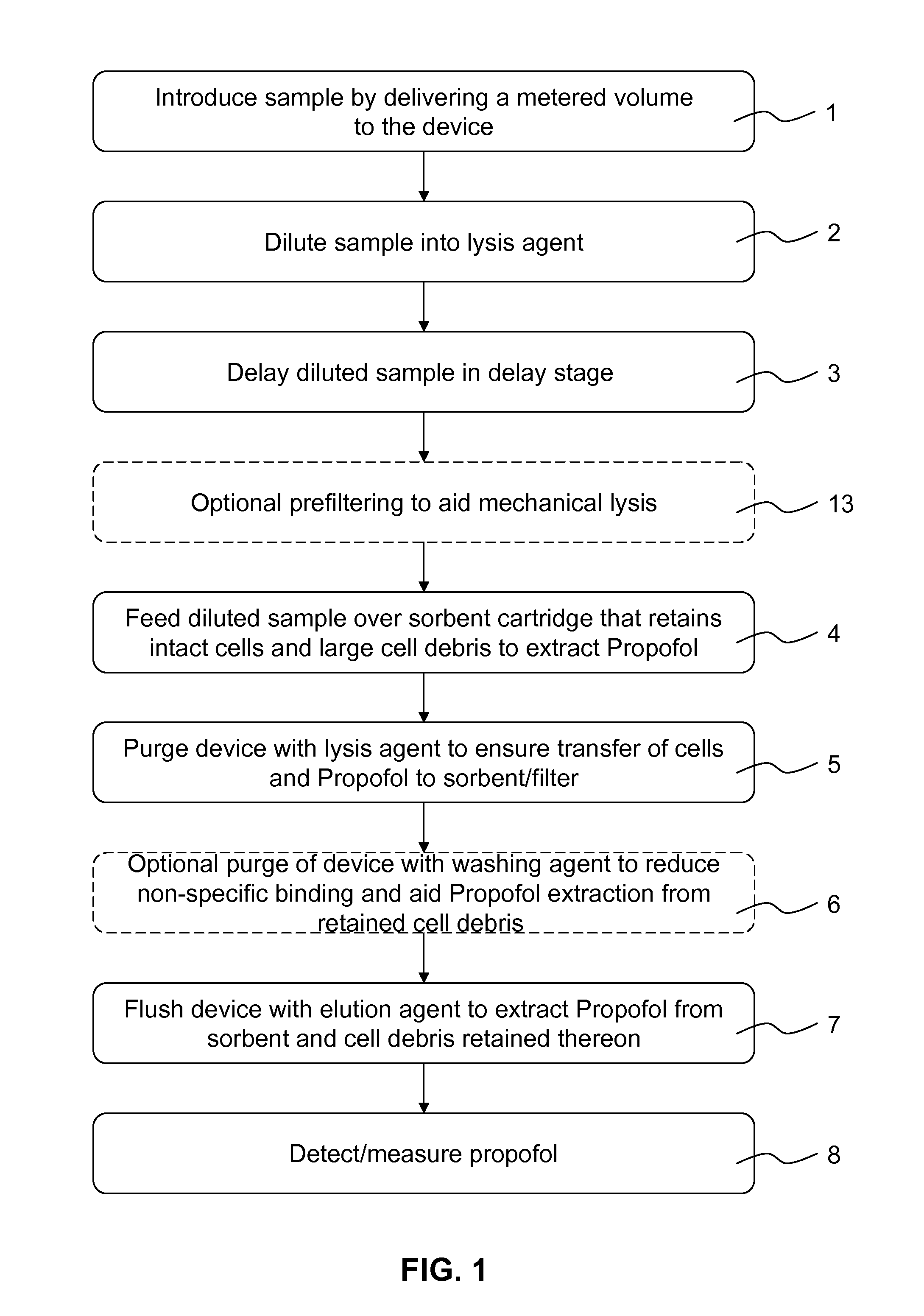
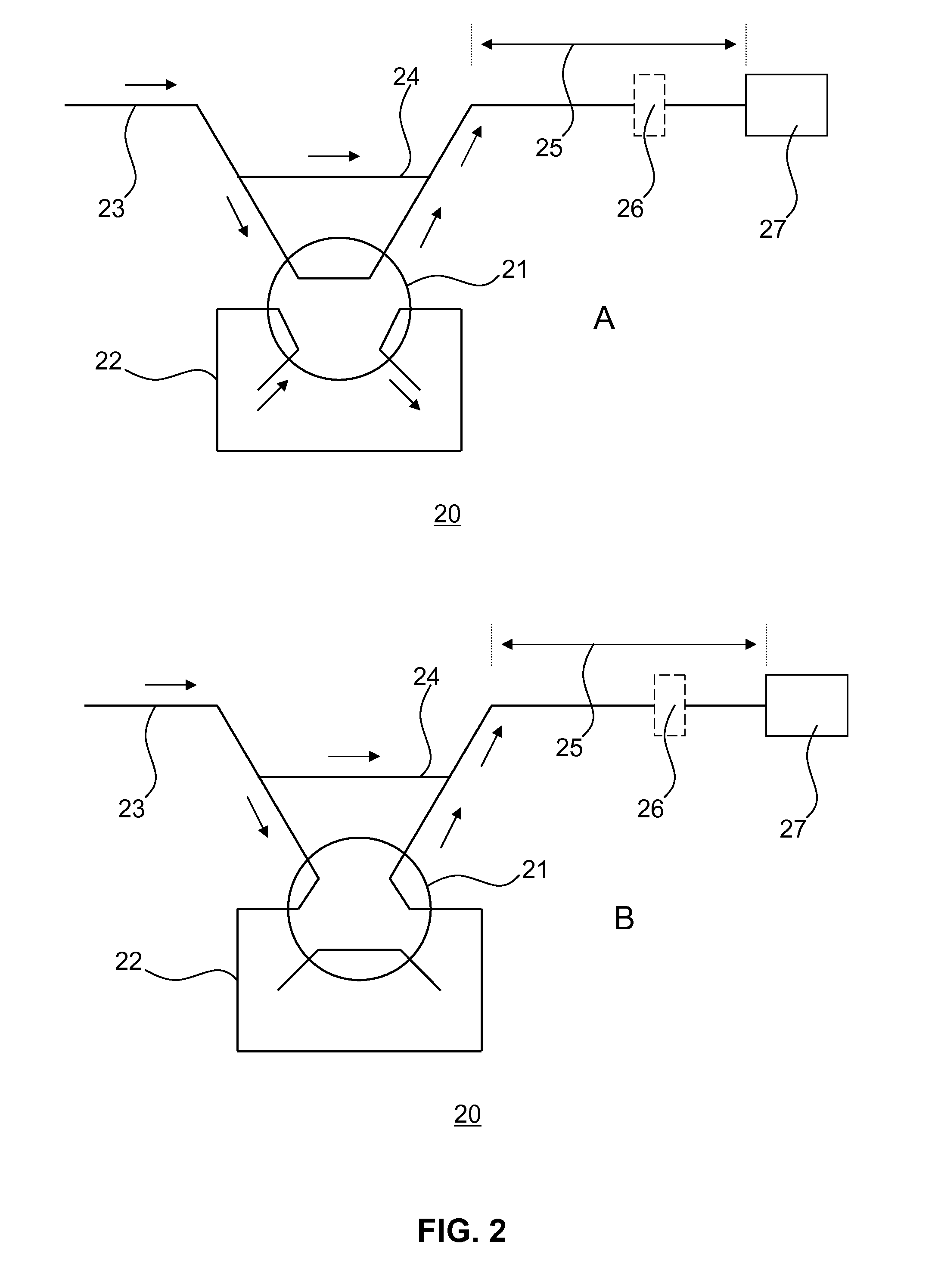
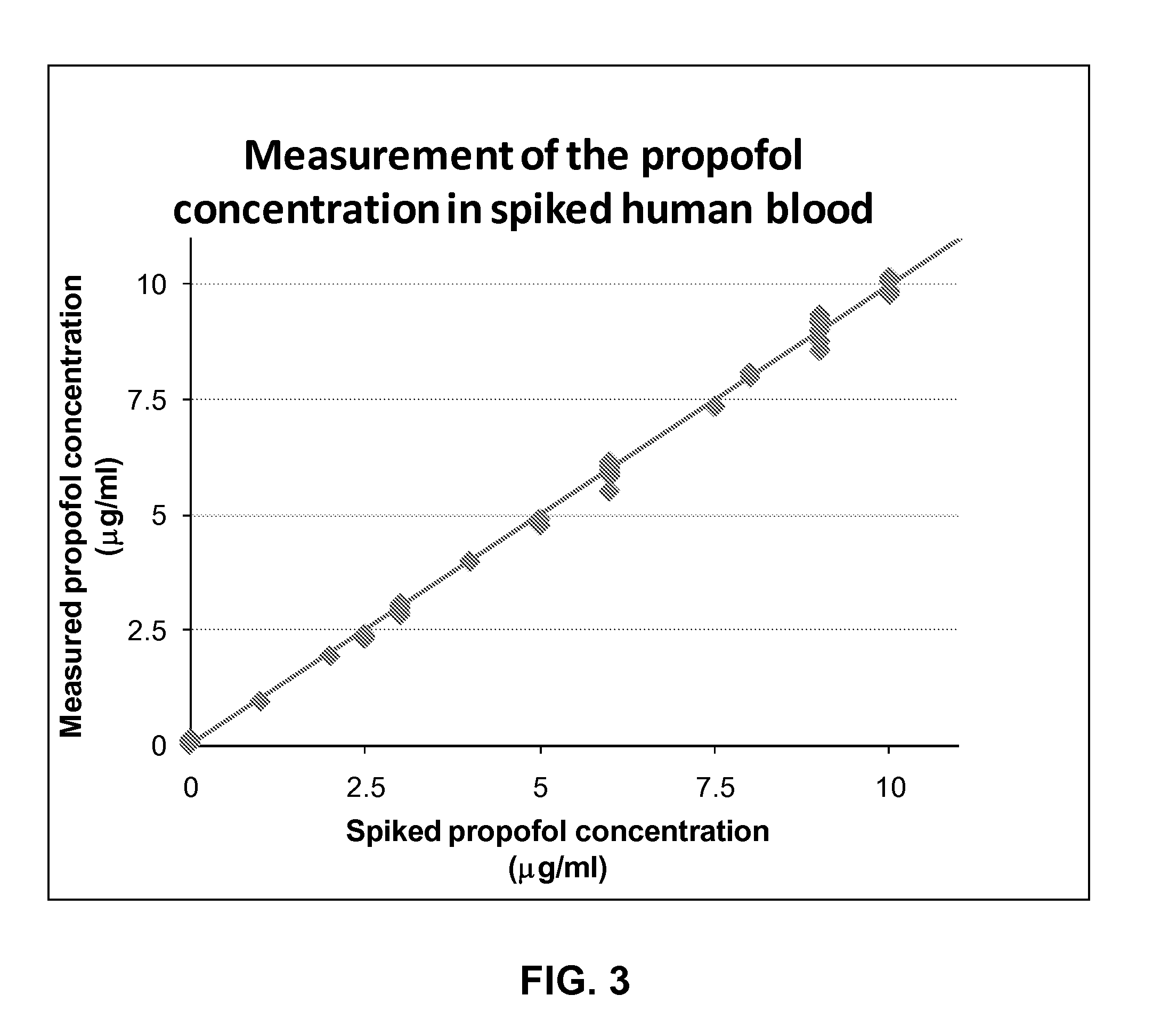
Analyte extraction apparatus and method
ActiveUS20130316464A1Improve accuracyEasy to useComponent separationBiological testingAnalyteSorbent
Disclosed is an apparatus for automatically extracting i2,6-diisopropylphenol (Propofol), from a complex sample matrix comprising cellular material, e.g. blood, the apparatus comprising a sample reception stage having on output for providing a defined quantity of the complex sample matrix; a mixing stage having a first input in fluidic connection with the output of the sample reception stage, a second input for receiving a lysing agent and an output for providing a mixture of the defined quantity of the complex sample matrix and the lysing agent; a delay stage having an input in fluidic connection with the output of the mixing stage and an output for providing the delayed mixture of the defined quantity of the complex sample matrix and the lysing agent; a filtering stage comprising a sorbent material for mechanically lysing the cellular material, said sorbent material having an affinity for binding Propofol, said filtering stage having an input in fluidic connection with the output of the delay stage; and a controller for controlling the flow rate of the mixture of the defined quantity of the complex sample matrix and the lysing agent through said delay stage. A method for such extraction is also disclosed.
Owner:SPHERE MEDICAL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com