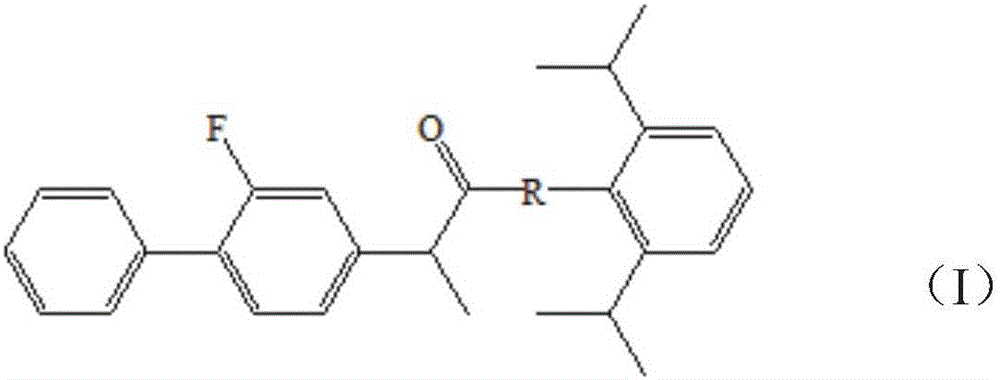
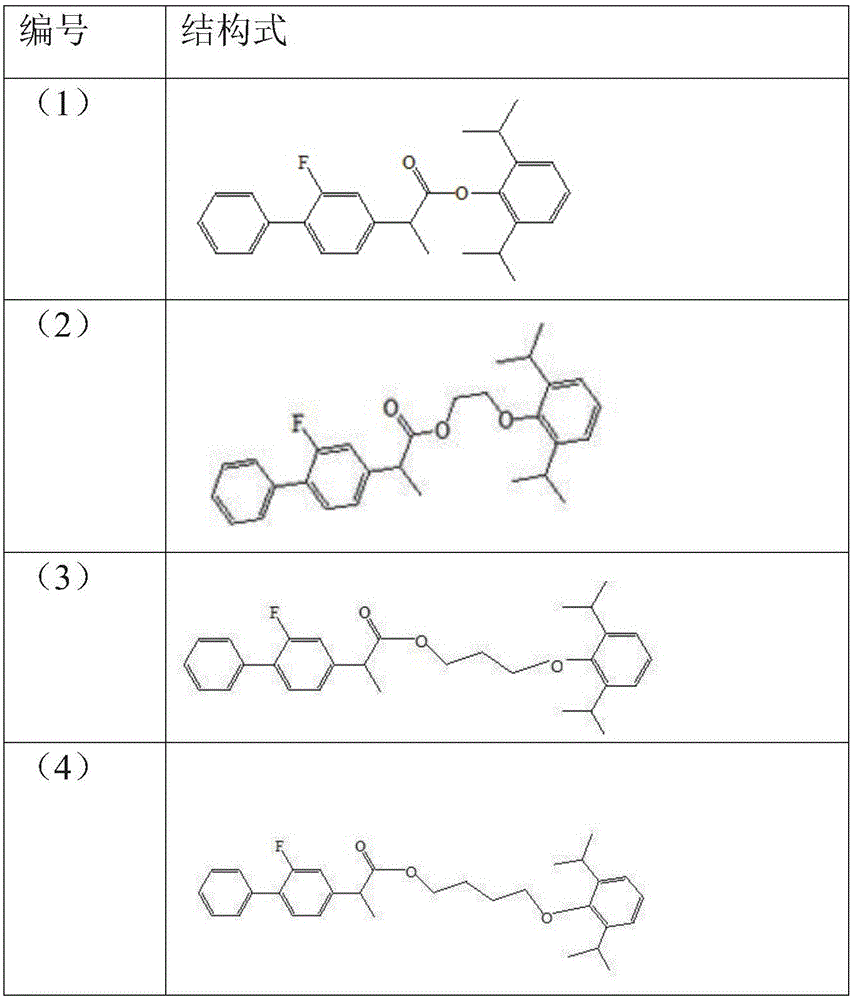
Double-effect anesthetic and preparation method and application thereof
A technology of drugs and compounds, applied in the field of medicine, can solve problems such as unpredictable drug interactions and complicated methods, and achieve the effects of strong intraoperative controllability, reduced incidence and severity, and quick recovery of patients.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
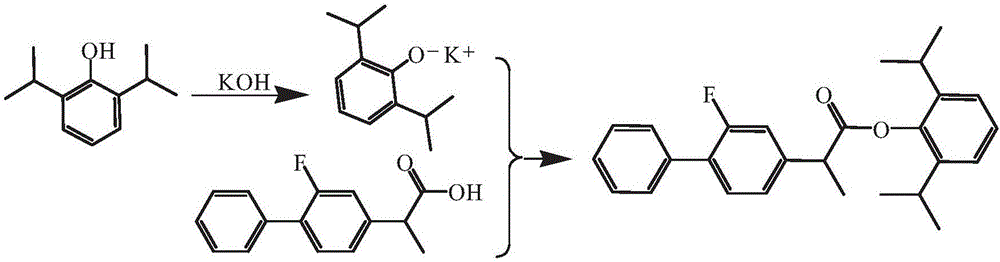
[0033] Embodiment 1: Flurbiprofen reacts with propofol potassium salt to prepare compound of the present invention (1)
[0034] A) Preparation of propofol potassium salt: add 24.9g propofol (0.14mol), 8.4g potassium hydroxide (0.15mol) and 300mL toluene into a 500mL three-necked flask, stir, heat up to 100°C for reflux reaction for 3h , using a water separator to continuously separate the water produced by the reaction. Stop the reaction when no more water comes out of the trap. The system was transferred to a single-necked bottle and concentrated under reduced pressure to remove the solvent toluene to obtain a light yellow crude product of propofol potassium salt. The crude product was stirred with 150 mL butanone for 8 min, the insoluble matter was removed by filtration, and the filtrate was concentrated and dried under reduced pressure to obtain light yellow propofol potassium salt;
[0035] B) Dissolve the above propofol potassium salt with 300mL acetonitrile, transfer t...
Embodiment 2
[0036] Embodiment 2: Compound of the present invention (1) prepared by the reaction of flurbiprofen acyl chloride and propofol
[0037] A) Preparation of flurbiprofen acyl chloride: get 53.7g flurbiprofen (0.22mol) into a 500mL dry three-necked flask, add 150mL dichloromethane, stir, and control the temperature in an ice bath at 0-5°C, preferably 3 ℃, slowly add 49.3mL of thionyl chloride dropwise, slowly warm up to room temperature after dropping, stir and react for 4 hours, and perform TLC reaction. After the reaction is completed, excess thionyl chloride is recovered under reduced pressure to obtain a light yellow oil;
[0038] B) Take 39.2g of propofol (0.22mol), 50mL of pyridine and 100mL of dichloromethane into a 500mL dry there-necked flask, stir; control the temperature at 0-10°C, preferably 5°C, put the shallow The yellow oil was dissolved in 150mL of dichloromethane and slowly added dropwise to the system. After the drop was completed, the temperature was raised to r...
Embodiment 3
[0039]Embodiment 3: Compound of the present invention (1) prepared by reacting flurbiprofen acyl bromide and propofol sodium salt
[0040] A) Add 34.2g flurbiprofen (0.14mol) into a 250mL dry there-necked flask, add 120mL tetrahydrofuran; stir; control the temperature in an ice bath at 0-5°C, preferably 3°C, and slowly add 20mL of phosphorus oxybromide dropwise , slowly warming up to room temperature after dropping, stirring and reacting for 3h, carrying out TLC reaction, recovering excessive phosphorus oxybromide under reduced pressure after the completion of the reaction to obtain light yellow oil, dissolving the above light yellow oil in 100mL dichloromethane to obtain Dichloromethane solution containing acid bromide;
[0041] B) Add 24.9g of propofol (0.14mol), 3.6g of sodium hydride (0.15mol) and 300mL of tetrahydrofuran into a 500mL three-necked flask, and stir at room temperature for 4 hours; after the above system is concentrated and dried under reduced pressure, add 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com