Preparation and application of monodisperse polyethylene glycol monomethyl ether modified propofol prodrug
A technology of polyethylene glycol monomethyl ether and propofol, applied in the field of preparation and application of propofol prodrugs, can solve the problems of aggravating hypotension, transient apnea, short shelf life, injection pain and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 3
[0056] Embodiment 3 Solubility Measurement Experiment
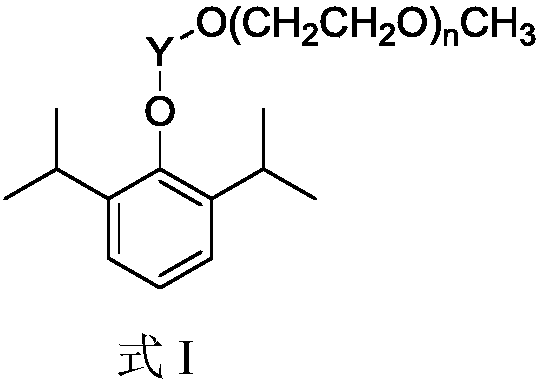
[0057] The solubility of compounds 1a-1f and 2a-2f in PBS buffer (pH=7.4) was determined by UV spectrophotometry. First, measure the UV absorbance of solutions with different concentrations of the compound at a wavelength of 257 nm, draw a standard curve to obtain a regression curve equation, then prepare a saturated solution of the compound, centrifuge the saturated solution and dilute the supernatant to a certain concentration, and then measure its concentration in The wavelength is the absorbance value at 257nm, which is brought into the standard curve equation to obtain the diluted concentration of the saturated solution of the compound, and then multiplied by the dilution factor to obtain the solubility of the compound in PBS buffer (pH=7.4). The solubility measurement results are shown in Table 1. The longer the chain of monodisperse polyethylene glycol monomethyl ether, the better the solubility of propofol prodrug...
Embodiment 4
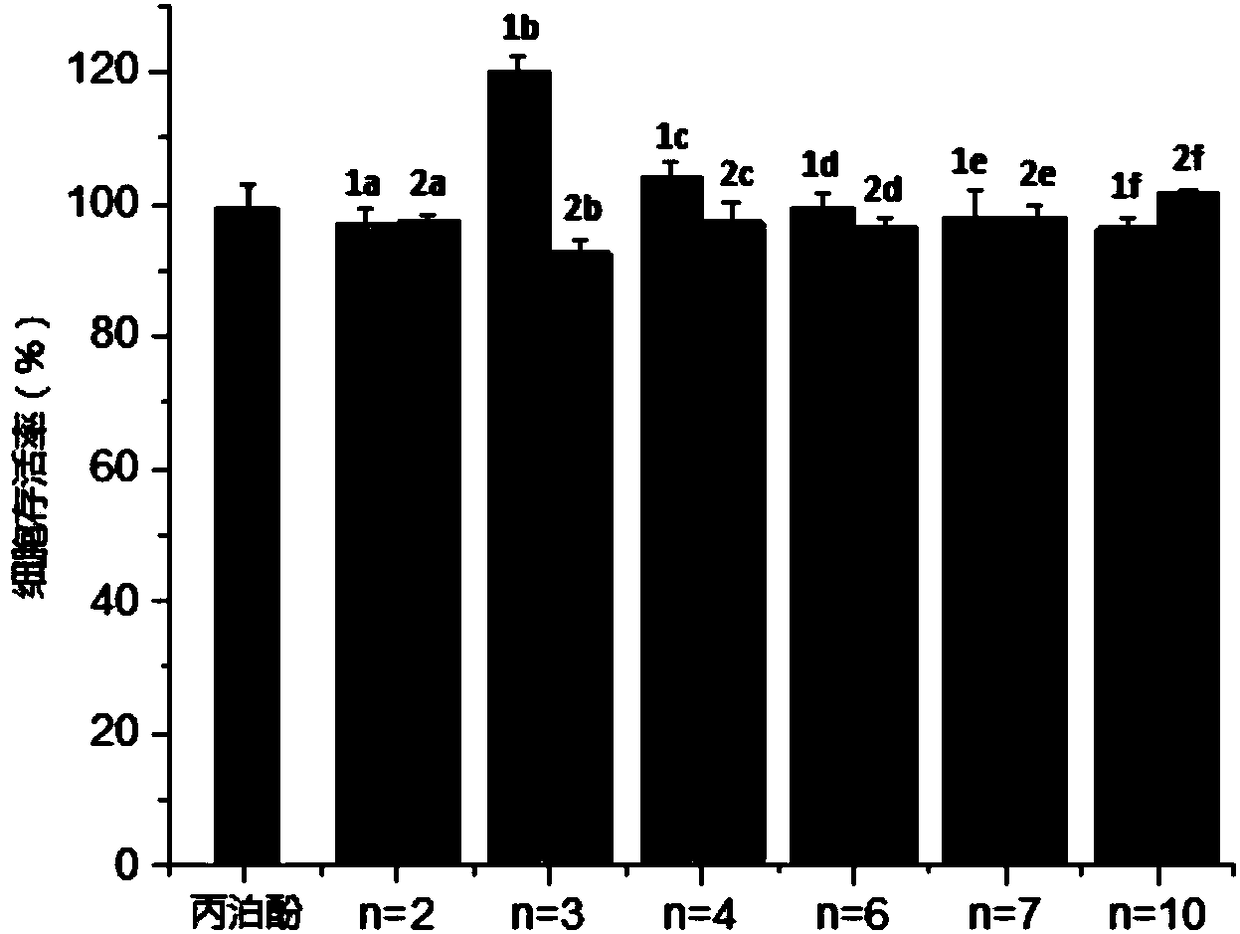
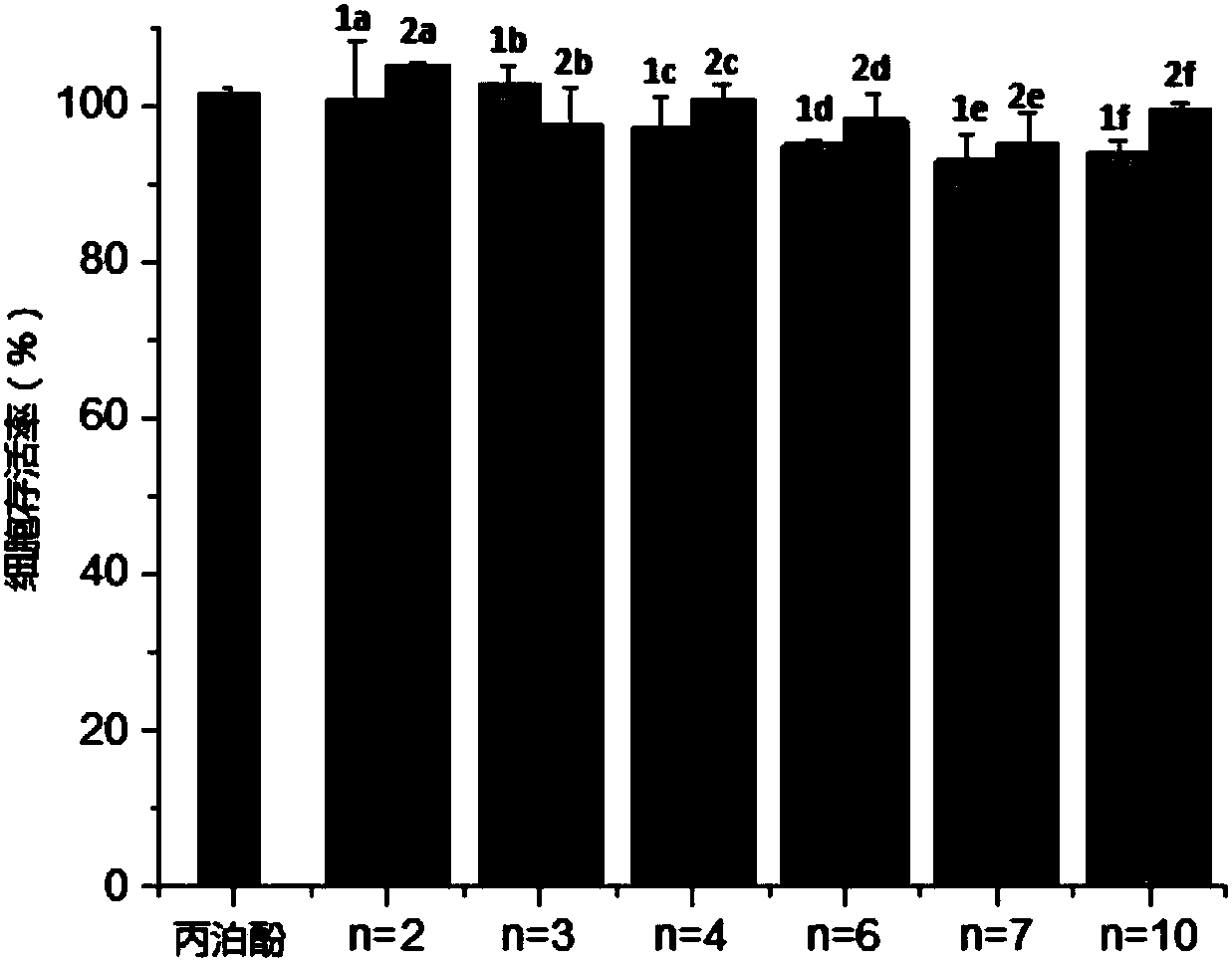
[0061] Example 4 Cell Viability Experiment
[0062] (1) Preparation of solution
[0063] Preparation of DMEM high glucose medium: buy DMEM high glucose medium, 500mL per bottle, add 10% fetal bovine serum and 1% penicillin-streptomycin solution, that is, add 50mL fetal bovine serum and 5mL to each bottle of medium of penicillin-streptomycin solution, the preparation of the medium was carried out in an ultra-clean workbench, and the prepared DMEM high-glucose medium was placed in a refrigerator at 4°C for storage.
[0064] Preparation of PBS buffer: In a 1000 mL conical flask, weigh 8 g of sodium chloride, 0.2 g of potassium chloride, 2.9 g of disodium hydrogen phosphate dodecahydrate, and 0.2 g of potassium dihydrogen phosphate, add 800 mL of pure water and stir to dissolve Then, the volume was adjusted to 1000 mL, and then stored in the refrigerator at 4°C after autoclaving.
[0065] Preparation of MTT solution: Weigh 0.5 g of MTT dry powder, dissolve it in 100 mL of PBS bu...
Embodiment 5
[0086] Example 5 Animal Anesthesia Experiment
[0087]Only the good water-soluble propofol prodrugs shown by the solubility measurement results are investigated here. Twelve healthy SD rats, weighing 180-200 g, were selected and randomly divided into five groups, with 3 rats in each group, and were raised under normal conditions before the test and during the observation period. Compounds 1f, 2e and 2f with good water solubility were respectively dissolved in physiological saline for injection. The commercially available propofol emulsion was used as a reference substance, and the doses were all measured according to the effective dose of propofol 13 mg / kg (0.073 mmol / kg). Tail vein injection was used to observe the phenomenon of anesthesia in the rats, and the duration of anesthesia was recorded. The experimental results are shown in Table 2.
[0088] Table 2 Anesthesia effect of different compounds on rats
[0089] compound
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com