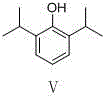
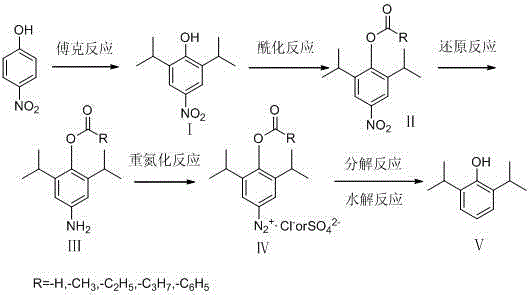
Preparation method of propofol
A technology of propofol and isopropanol, which is applied in the novel preparation field of intravenous anesthetic propofol (Propofol), can solve the problems of harsh reaction conditions, severe reaction, difficulty in controlling, many by-products, etc. The effect of novel route and easy product
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1 2,6-diisopropyl-4-nitrophenol (compound ) preparation
[0022] Slowly add 36 mL of concentrated sulfuric acid into 2.5 mL of cold water, and after cooling to room temperature, slowly add the solution dropwise to p-nitrophenol (10 g, 0.072 mol) in isopropanol (12 mL, 0.16 mol) in an ice bath In the solution, keep the temperature not exceeding 10°C during the dropwise addition, and raise the temperature to 60°C for 3 hours after the dropwise addition. Pour the reaction solution into 500 g of ice, adjust the pH to 11-13 with 20% NaOH solution, filter, wash the filtrate with petroleum ether, then adjust the pH of the water layer to 3-4 with 2mol / L HCl, precipitate solids, filter with suction, collect and filter The cake was vacuum-dried to obtain 14.3 g of the product (yield: 89.1%). 1 H-NMR (CDCl 3 ): δ 7.99 (s, 2H), 3.20 (m, 2H), 1.31 (d, 12H).
Embodiment 2
[0023] Example 2 2,6-diisopropyl-4-nitrophenol (compound ) preparation
[0024] Add 36 mL of 85% phosphoric acid to a 100 mL three-neck flask, add p-nitrophenol (10 g, 0.072 mol), stir in an ice bath, and slowly add isopropanol (12 mL, 0.16 mol) dropwise. Keep the temperature at no more than 10°C, and raise the temperature to 60°C for 3 hours after the dropwise addition. Pour the reaction solution into 500 g of ice, adjust the pH to 11-13 with 20% NaOH solution, filter, wash the filtrate with petroleum ether, and then adjust the pH of the water layer to 3-4 with 2 mol / L HCl, precipitate solid, suction filter, collect and filter The cake was vacuum-dried to obtain 13.3 g of the product (yield: 82.9%).
Embodiment 3
[0025] Example 3 2,6-diisopropyl-4-nitrophenol (compound ) preparation
[0026] Add 50 mL of 40% hydrofluoric acid into a three-necked flask, add p-nitrophenol (10 g, 0.072 mol), stir in an ice bath, and slowly add isopropanol (12 mL, 0.16 mol) dropwise. Keep the temperature at no more than 10°C, and raise the temperature to 60°C for 3 hours after the dropwise addition. Pour the reaction solution into 500 g of ice, adjust the pH to 11-13 with 20% NaOH solution, filter, wash the filtrate with petroleum ether, and then adjust the pH of the water layer to 3-4 with 2 mol / L HCl, precipitate solid, suction filter, collect and filter The cake was vacuum-dried to obtain 11.3 g of the product (yield: 70.4%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com