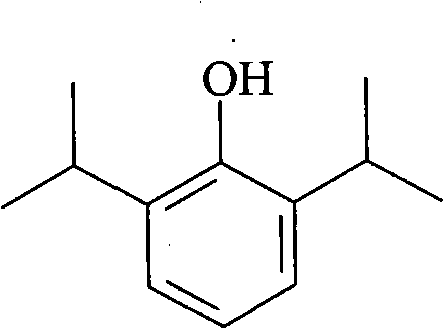
Formula and preparation method of novel propofol fat emulsion preparation causing no pain and low injection stimulation
A technology of propofol fat and propofol, which can be used in oil/fat/wax inactive ingredients, anesthetics, emulsion delivery, etc., and can solve problems such as in-depth research on prostaglandins
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
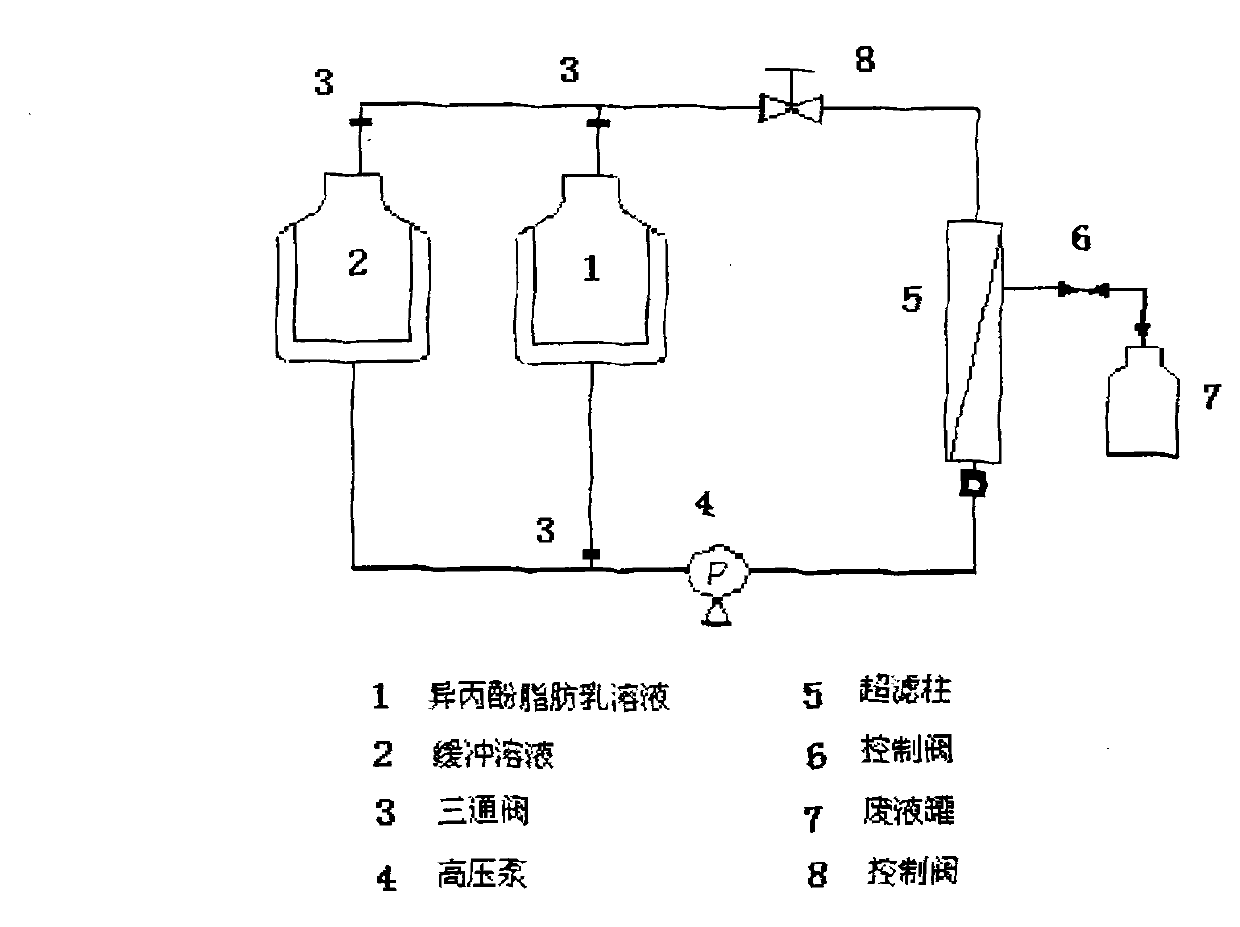
[0084] Example 1: Investigation of the applicability of ultrafiltration to ordinary propofol fat emulsion
[0085] Get 1000mL of propofol fat emulsion (such as the sample prepared according to Example 2) prepared by any embodiment, and take a small amount of sample to analyze the free propofol concentration in its water phase. according to figure 2 Assemble the "ultrafiltration production equipment" and select the ultrafiltration cartridge with suitable pore size. The main factor in the selection is that the aqueous solution, especially the free propofol in the water phase, can pass freely, while the fat emulsion cannot pass through as the selection parameter. First, equilibrate the ultrafiltration column with isotonic buffer solution, then slowly input the propofol fat emulsion into the ultrafiltration column to adjust the column pressure. The aqueous solution containing free propofol passes through the ultrafiltration column and is effectively separated from the propofol f...
Embodiment 2
[0086] Example 2: Investigation of the applicability of ultrafiltration to polyethylene glycol propofol fat emulsion
[0087] Get the polyethylene glycol propofol fat emulsion sample 1000mL prepared according to Example 10, take a small amount and analyze the free propofol concentration in its water phase. according to figure 2 Assemble the "ultrafiltration production equipment", select the ultrafiltration cartridge with the appropriate pore size, and use the water solution to pass through freely, but the fat emulsion cannot pass through as the selection parameter. First, equilibrate the ultrafiltration column with isotonic buffer solution, then slowly input the propofol fat emulsion into the ultrafiltration column to adjust the column pressure. The aqueous solution containing free propofol passes through the ultrafiltration column and is effectively separated from the propofol fat emulsion. The missing solution is replenished by buffer solution. Generally, the free propof...
Embodiment 3
[0092] Example 3: Highest propofol to diluent oil ratio (propofol: soybean oil weight ratio is 0.07)
[0093] prescription:
[0094] Propofol 10.0g
[0095] Soybean oil (for injection) 143.0g
[0096] Lecithin (for injection) 12.5g
[0097] Vitamin E 3.0g
[0098] Glycerin 22.0g
[0099] Add water for injection to 1000ml
[0100] Take 10.0g of propofol, 3.0g of vitamin E, 143.0g of refined soybean oil, add 12.5g of lecithin, heat and stir for about 10-20min to mix well. Another 700ml of water for injection was taken, and 22.0g of glycerin was added. Under nitrogen protection and stirring conditions, the propofol phospholipid oil solution was added to the aqueous glycerin solution, and the total amount was adjusted to 1000ml. Homogenize 7-8 times with a high-pressure homogenizer, the homogenization pressure is 100MPa, until the particle size is less than 300nm, adjust the pH to 6.0-8.0, filter, subpackage, inject nitrogen, and seal. Sterilize at 115°C for 15 minutes, an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com