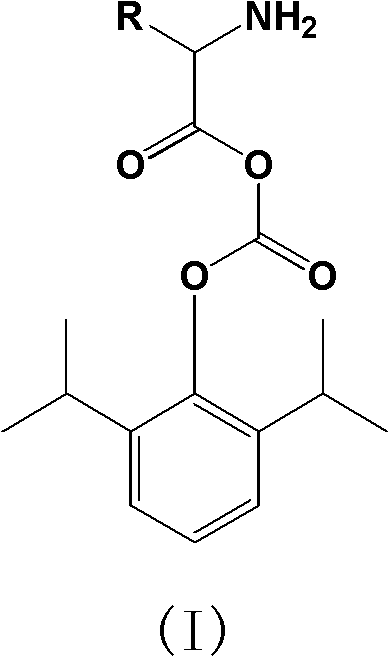
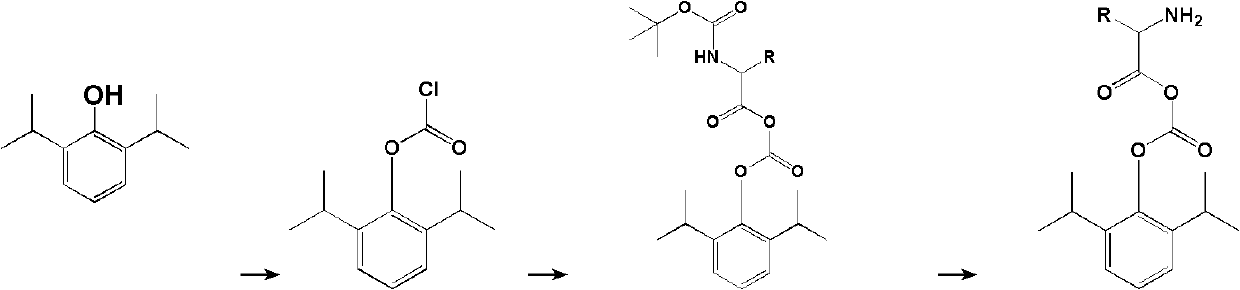
Carbonic acid diester water-soluble derivant of amino acid and propofol, and application of carbonic acid diester water-soluble derivant of amino acid and propofol
A technology of amino acids and derivatives, which is applied in the field of water-soluble derivatives of carbonate diesters of amino acids and propofol and their uses, and can solve problems such as unfavorable drug safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] Synthesis of 2-L-lysine-(2,6-diisopropyl-phenyl-)carbonic anhydride dihydrochloride (compound 1)
[0072]
[0073] Compound 1
[0074] Under water bath conditions, propofol (2.6mmol, 0.46g) was added to anhydrous dichloromethane (30ml), cooled to -5°C, triphosgene (0.9mmol, 0.27g) was added, and under temperature control, dropwise Add triethylamine (2.6 mmol, 0.26 g), slowly warm up to room temperature, and stir for 1 hour. then add N α -tert-butoxycarbonyl-N ε -tert-butoxycarbonyl-lysine (2.2mmol, 0.76g), triethylamine (2.2mmol, 0.22g), stirred overnight at room temperature. The reaction was monitored by HPLC until the raw material was completely reacted. Concentrate, add 4M HCl / dioxane solution (20ml), stir and react at room temperature for 1h. The solvent was evaporated under reduced pressure, the insoluble matter was washed by adding n-hexane, and filtered to obtain a white solid, which was recrystallized from ether / n-hexane to obtain 0.61 g of compound 1 ...
Embodiment 2
[0079] Synthesis of 2-L-arginine-(2,6-diisopropyl-phenyl-)carbonic anhydride dihydrochloride (compound 2)
[0080]
[0081] Compound 2
[0082] Synthetic method with reference to embodiment 1, with N α - tert-butoxycarbonyl-arginine hydrochloride replaces N α tert-butoxycarbonyl-N ε - tert-butoxycarbonyl-lysine. Compound 2 was obtained with a yield of 60.1%.
[0083] ESI-MS m / z: 379 (M+1 + ).
[0084] 1 H NMR (D 2 O)δ0.98(d,12H,-C H 3 ), 1.6-1.7 (m, 2H, CH 2 -C H 2 -CH 2 - δ NH-), 1.8-2.0 (m, 1H, C H), 2.2-2.2 (m, 1H, C H ), 2.99(t, 2H, C H 2 -CH 2 -CH 2 - δ NH-), 3.2-3.3 (m, 2H, CH 2 -C H 2 -δNH-), 4.41 (dd, 1H, C H - α NH 2 ), 7.1-7.3 (m, 3H, Ar H ).
[0085] Elemental analysis: calculated value C, 50.56; H, 7.15; N, 12.41; found value 50.39; H, 7.11; N.12.50
Embodiment 3
[0087] Synthesis of 2-glycine-(2,6-diisopropyl-phenyl-)carbonic anhydride hydrochloride (compound 3)
[0088]
[0089] Compound 3
[0090] Synthetic method with reference to embodiment 1, with N α tert-butoxycarbonyl-glycine replaces N α tert-butoxycarbonyl-N ε - tert-butoxycarbonyl-lysine. Compound 3 was obtained with a yield of 58.3%.
[0091] ESI-MS m / z: 280 (M+1 + ).
[0092] 1 H NMR (D 2 O)δ0.99(d,12H,-C H 3 ), 1.8-2.0 (m, 1H, C H ), 2.2-2.2 (m, 1H, C H ), 4.39 (dd, 1H, C H - α NH 2 ), 7.1-7.3 (m, 3H, Ar H ).
[0093] Elemental analysis: calculated value C, 57.05; H, 7.02; N, 4.44; found value 57.21; H, 7.09; N.4.29.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com