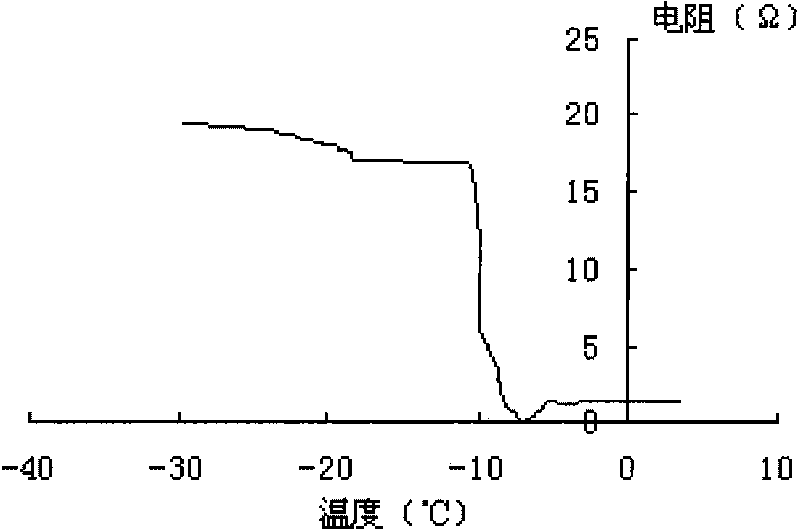
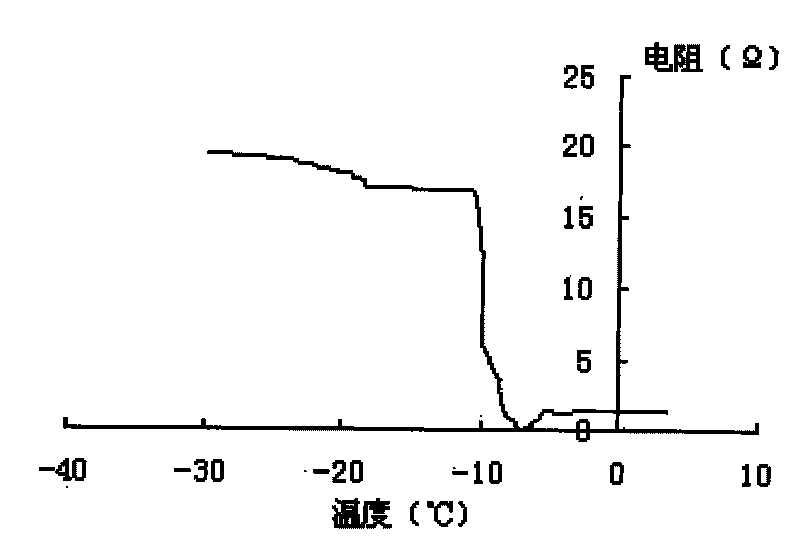
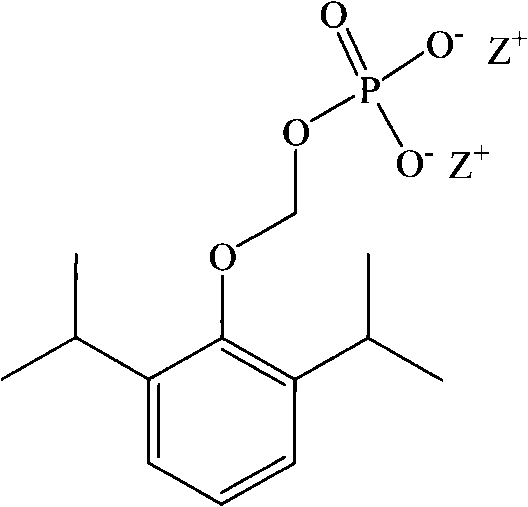
New precursor medicinal preparation
A preparation and liquid medicine technology, which is applied in the field of freeze-dried preparations and preparations of propofol water-soluble precursor compounds, can solve instability and other problems, and achieve long-term stable storage and high safety effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Example 1 Freeze-dried powder injection of compound of formula I (Z is sodium)
[0058] Prescription: single dose
[0059] Formula I compound (Z is sodium) 0.1g
[0060] Mannitol 0.025g
[0061] Appropriate amount of water for injection
[0062] The preparation method is as follows: weighing according to the formula, dissolving the compound of formula I (Z is sodium) in water for injection, adding mannitol, and stirring until completely dissolved. The amount of activated carbon added is 0.05-1.0% (w / v), stirred for 30 minutes, and filtered. The filtrate is sterilized by filtration through a 0.22um microporous membrane, filled in vials or vials, half-stoppered, placed on the shelf of a freeze dryer, pre-frozen at -30~-50°C for 1 to 4 hours, and then set the temperature Dry once at -10-0°C for 8-24 hours, and continue drying at 20-30°C for 2-12 hours until the moisture reaches the requirement. Vacuum or nitrogen-filled pressure plug is enough, the sample is redissolv...
Embodiment 2
[0063] Example 2 Freeze-dried powder injection of the compound of formula I (Z is sodium) without excipients
[0064] Prescription: single dose
[0065] Formula I compound (Z is sodium) 0.1g
[0066] Appropriate amount of water for injection
[0067] The preparation method is: weighing according to the formula, dissolving the compound of formula I (Z is sodium) in water for injection, and stirring until completely dissolved. The amount of activated carbon added is 0.05-1.0% (w / v), stirred for 30 minutes, and filtered. The filtrate is sterilized by filtration through a 0.22um microporous membrane, filled in vials or vials, half-stoppered, placed on the shelf of a freeze dryer, pre-frozen at -30~-50°C for 1 to 4 hours, and then set the temperature Dry once at -10-0°C for 8-24 hours, and continue drying at 20-30°C for 2-12 hours until the moisture reaches the requirement. Vacuum or nitrogen-filled pressure plug is enough, the sample is redissolved quickly and the solution is ...
Embodiment 3
[0068] Example 3 Compatibility test of compound of formula I (Z is sodium) and common lyophilized excipients
[0069] With reference to the "Technical Guidelines for the Research of Chemical Drug Preparations" and the "Chinese Pharmacopoeia" 2005 edition two appendix stability inspection methods, the phase of the formula I compound (Z is sodium) and the common lyophilized excipients was determined by high performance liquid phase method. Capacitance.
[0070] The following table is the measurement result of formula I compound (Z is sodium) and sodium chloride, mannitol under different conditions
[0071]
PUM
| Property | Measurement | Unit |
|---|---|---|
| solubility (mass) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com