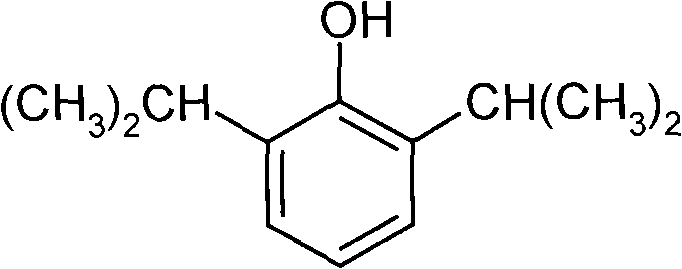
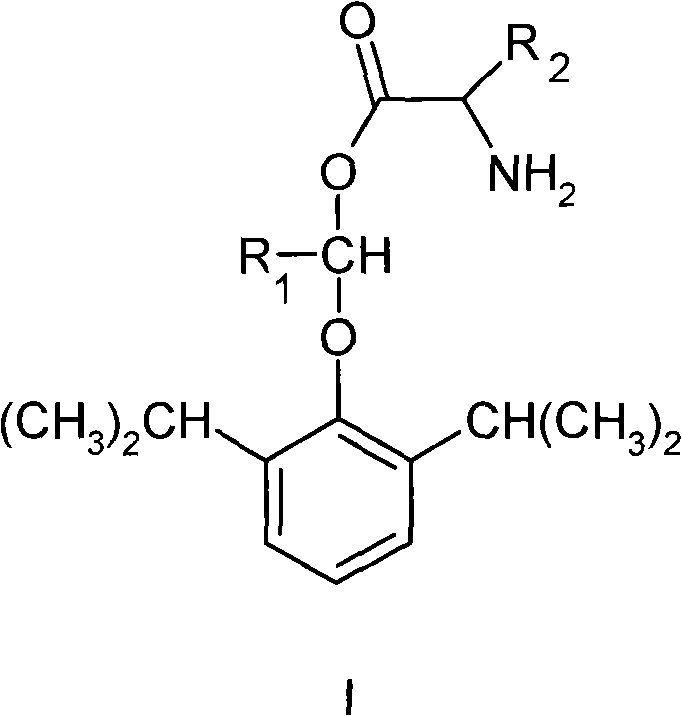
Water-soluble amino-acid ester derivative of propofol
A technology of amino acid esters and derivatives, which can be used in drug combinations, anesthetics, organic chemistry, etc., and can solve problems such as difficult to use, easy to grow bacteria, poor physical stability of emulsions, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 12
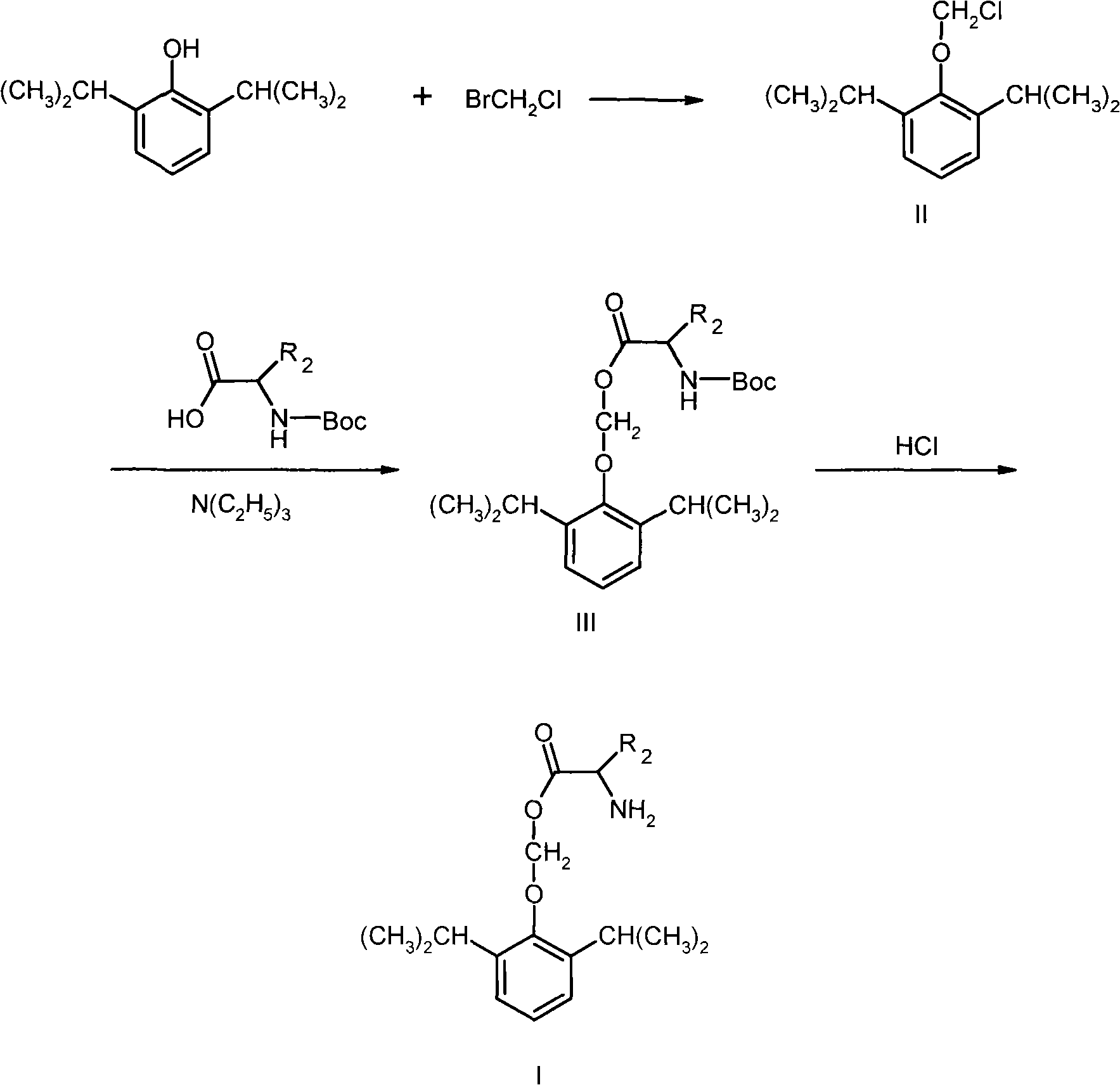
[0024] Example 12-(L-glycyloxymethyloxy)-1,3-diisopropyl-benzene hydrochloride (I 1 ) preparation
[0025]
[0026] 1. Synthesis of 12-chloromethyloxy-1,3-diisopropyl-benzene
[0027] Dissolve 30.0g propofol in 500ml dry tetrahydrofuran under N 2 12g of sodium hydroxide and 380g of bromochloromethane were added under protection. The reaction was stirred at 64°C for 3 hours, cooled to room temperature, filtered, and the filtrate was evaporated to dryness under reduced pressure to obtain 31 g of 2-chloromethyloxy-1,3-diisopropyl-benzene. Proton NMR spectrum δ (ppm, CDCl3): 7.21-7.08 (m, 3H); 5.76 (s, 2H); 3.36 (m, 2H); 1.21 (d, 12H). 1.22-(L-glycyloxymethyloxy)-1,3-diisopropyl-benzene hydrochloride (I 1 )Synthesis
[0028] in N 2 Under protection, add 2.1g N-Boc-L-glycine, 1.7ml milliliter triethylamine, 1.2g 2-chloromethyloxy-1,3-diisopropyl-benzene to 30ml acetonitrile, stir at 60°C Reacted for 10 hours, after cooling, the solid was filtered off, the filtrate was con...
Embodiment 2
[0030] Example 22-(L-alanyloxymethyloxy)-1,3-diisopropyl-benzene hydrochloride (I 2 ) preparation
[0031]
[0032] With reference to the method of Example 1.2, replace N-Boc-L-glycine with N-Boc-L-alanine and react with 2-chloromethyloxy-1,3-diisopropyl-benzene to obtain 2-( N-Boc-L-alanyloxymethyloxy)-1,3-diisopropyl-benzene. Proton NMR spectrum δ (ppm, CDCl3): 7.20-7.09 (m, 3H); 5.48 (s, 2H); 4.31 (q, 1H); 3.35 (m, 2H); 1.60 (d, 3H); 1.40 ( s, 9H); 1.22 (d, 12H).
[0033] Deprotection of 2-(N-Boc-L-alanyloxymethyloxy)-1,3-diisopropyl-benzene with hydrogen chloride affords I 2 . Proton NMR spectrum δ (ppm, DMSO-d6): 8.25 (br s, 2H); 7.22-7.11 (m, 3H); 5.46 (s, 2H); 4.35 (q, 1H); 3.38 (m, 2H) ; 1.62(d, 3H); 1.22(d, 12H).
Embodiment 32
[0034] Example 32-(L-valyloxymethyloxy)-1,3-diisopropyl-benzene hydrochloride (I 3 ) preparation
[0035]
[0036] With reference to the method of Example 1.2, replace N-Boc-L-glycine with N-Boc-L-valine and react with 2-chloromethyloxy-1,3-diisopropyl-benzene to obtain 2-( N-Boc-L-valyloxymethyloxy)-1,3-diisopropyl-benzene. Proton NMR spectrum δ (ppm, CDCl3): 7.20-7.09 (m, 3H); 5.44 (s, 2H); 4.28 (d, 1H); 3.35 (m, 2H); 2.36 (m, 1H); 1.40 ( s, 9H); 1.22 (d, 12H); 1.06 (d, 6H).
[0037] Deprotection of 2-(N-Boc-L-valyloxymethyloxy)-1,3-diisopropyl-benzene with hydrogen chloride affords I 3 . Proton NMR spectrum δ (ppm, DMSO-d6): 8.23 (br s, 2H); 7.15-7.04 (m, 3H); 5.43 (s, 2H); 4.23 (d, 1H); 3.31 (m, 2H) ; 2.32 (m, 1H); 1.19 (d, 12H); 0.96 (d, 6H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com