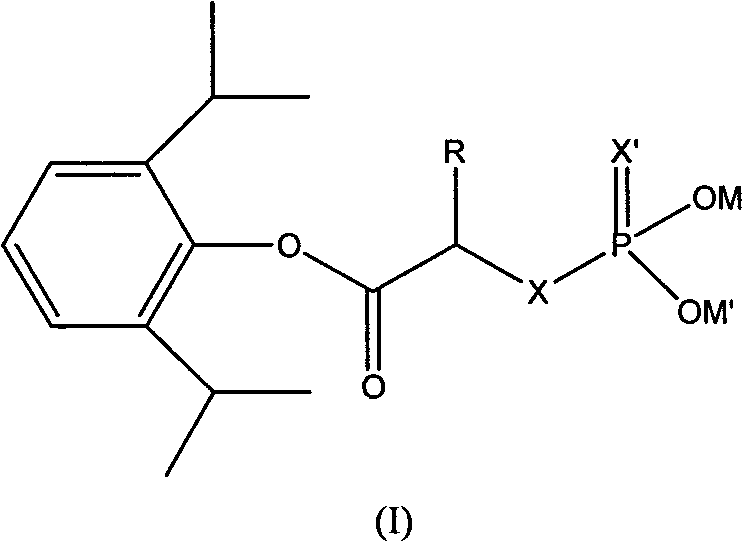
Phosphoryl carboxylic acid propofol ester derivative and preparation method thereof
A technology of phosphoryl carboxylic acid and phosphoryl mercaptocarboxylic acid, which is applied in phosphorous organic compounds, chemical instruments and methods, drug combinations, etc., can solve the problem of high industrialization cost, difficult preparation method of water-soluble prodrug, and low yield and other problems, to achieve the effect of fast onset, reduction or removal of injection pain, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Synthesis of Propofol 2-Chloroacetate
[0036]
[0037] method one:
[0038] In a 500ml four-neck round-bottomed flask, install a mechanical stirrer, a nitrogen gas introduction device, a condenser tube and a thermometer, add 8.9g (0.05mol) 2,6-diisopropylphenol, 100mlTHF, 2.4g (0.1 mol) sodium hydride, and the reaction solution was stirred below 10°C until no bubbles were generated. (3.0mol, 225.88ml) chloroacetyl chloride was added dropwise to the reaction solution, followed by gas phase until the reaction was complete, the suspension was filtered, the filter cake was washed with THF, THF was concentrated under reduced pressure, and the remaining oil was vacuum (0-1 torr) , b.p=80°C) distillation to obtain propofol 2-chloroacetate.
[0039] Method Two:
[0040] Cool the chloroform solution of 33.88g (0.3mol) of chloroacetyl chloride with an ice bath to lower the temperature of the solution to below 5°C, then add the fine powder of 2,6-diisopropylsodium phenate i...
Embodiment 2
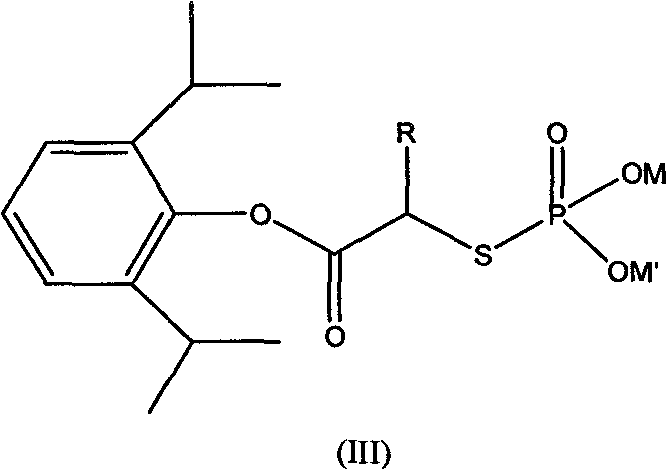
[0043] Synthesis of α-disodium phosphothioglycolate propofol
[0044]
[0045] In the three-necked flask, add sodium thiophosphate (72.03g, 0.2mol), propofol 2-chloroacetate (56.05g, 0.22mol) and 160ml of distilled water at one time, stir, and under ice water cooling, the temperature in the flask is natural Decrease to about 15°C, dropwise add 120ml of dimethyl sulfoxide (DMSO), the reaction temperature rises gradually, not exceeding 20°C, after the addition is complete, continue to stir until the reaction is complete to obtain a product solution; continue to add 95% ethanol dropwise to the solution 260ml, left for a while, overnight, to obtain a solid. Washed once with 45ml of alcohol, dried in a vacuum oven (30 inches Hg, 45° C.) for 48 hours to obtain a white solid α-disodium phosphothioglycolate propofol.
[0046] 1 H-NMR (D6-DMSO-D2O): δ1.29(d, 12H, 4CH3); 3.12(m, 2H, 2CH); 3.5(s, 2H, CH2); 6.91(t, H, CH); 7.31 (d, 2H, 2CH).
Embodiment 3
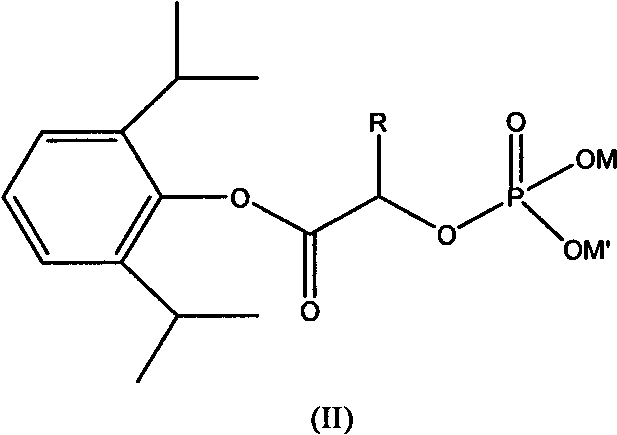
[0048] Synthesis of Propofol α-Disodium Phosphoryloxyacetate
[0049]
[0050] In a three-necked flask, add sodium phosphate (76.02g, 0.2mol), 2-propofol chloroacetate (56.05g, 0.22mol) and 160ml of distilled water at one time, stir, and under ice water cooling, the temperature in the flask naturally drops to At about 15°C, add 120ml of dimethyl sulfoxide (DMSO) dropwise, and the reaction temperature rises gradually, not exceeding 20°C. After the addition is complete, continue to stir until the reaction is complete to obtain a product solution; continue to add 260ml of 95% ethanol dropwise to the solution, Leave it for a while, overnight, to get a solid. Washed once with 45ml of alcohol, dried in a vacuum oven (30 inchesHg, 45° C.) for 48 hours to obtain a white solid α-disodium phosphooxyacetate propofol.
[0051] 1 H-NMR (D6-DMSO-D2O): δ1.29 (d, 12H, 4CH3,); 3.12 (m, 2H, 2CH); 4.97 (s, 2H, CH2); 6.91 (t, H, CH); 7.31 (d, 2H, 2CH).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com