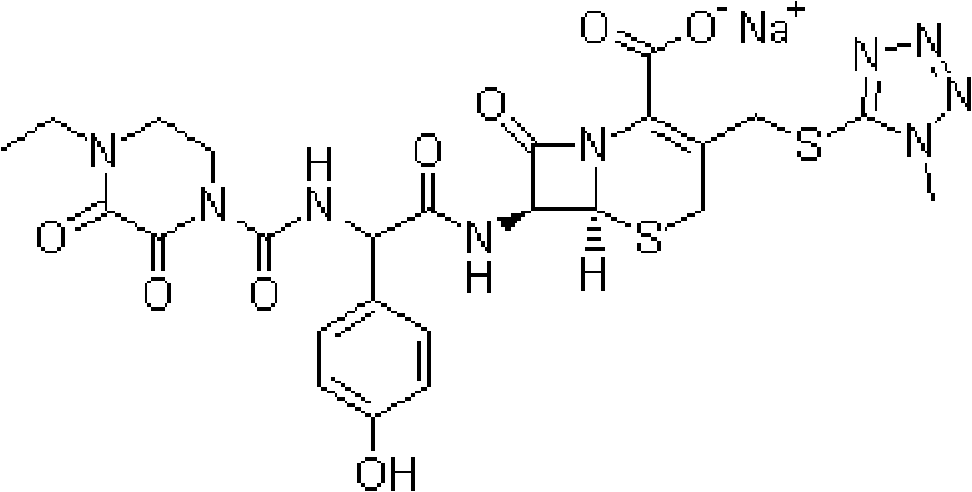
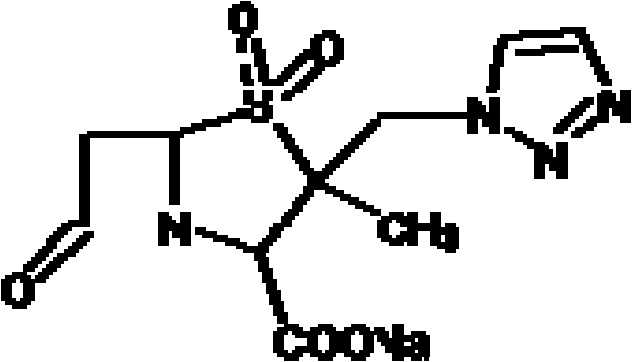
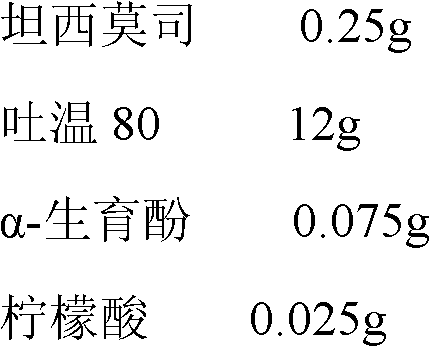
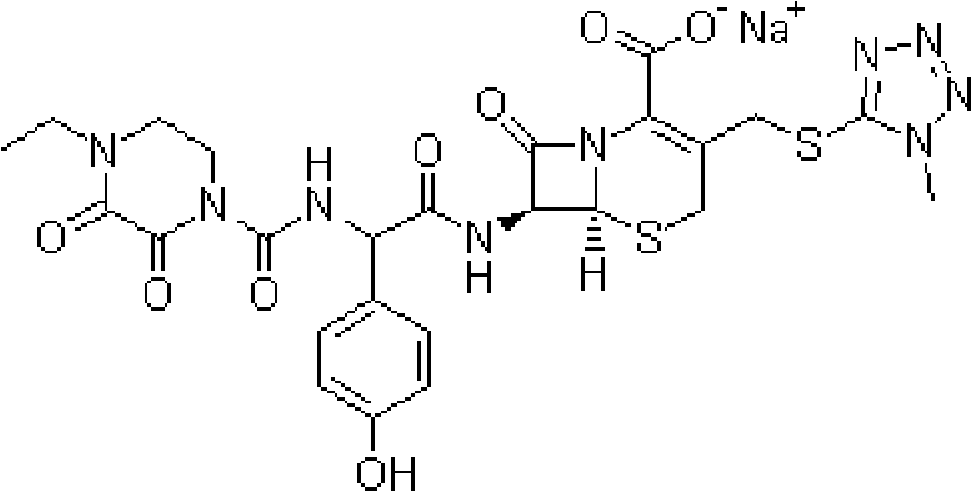
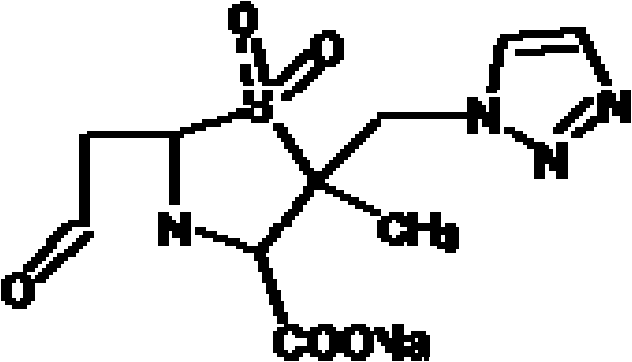
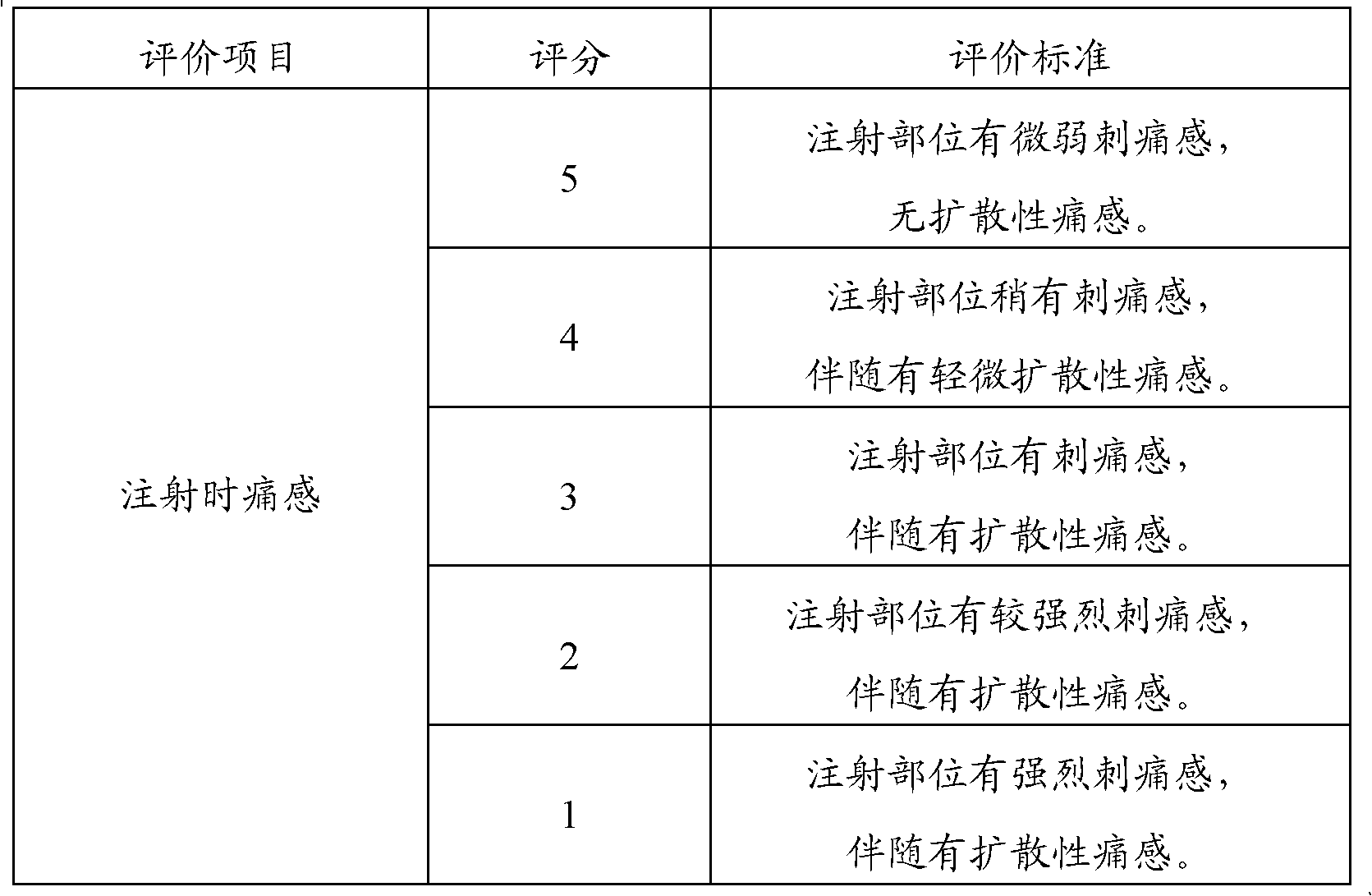
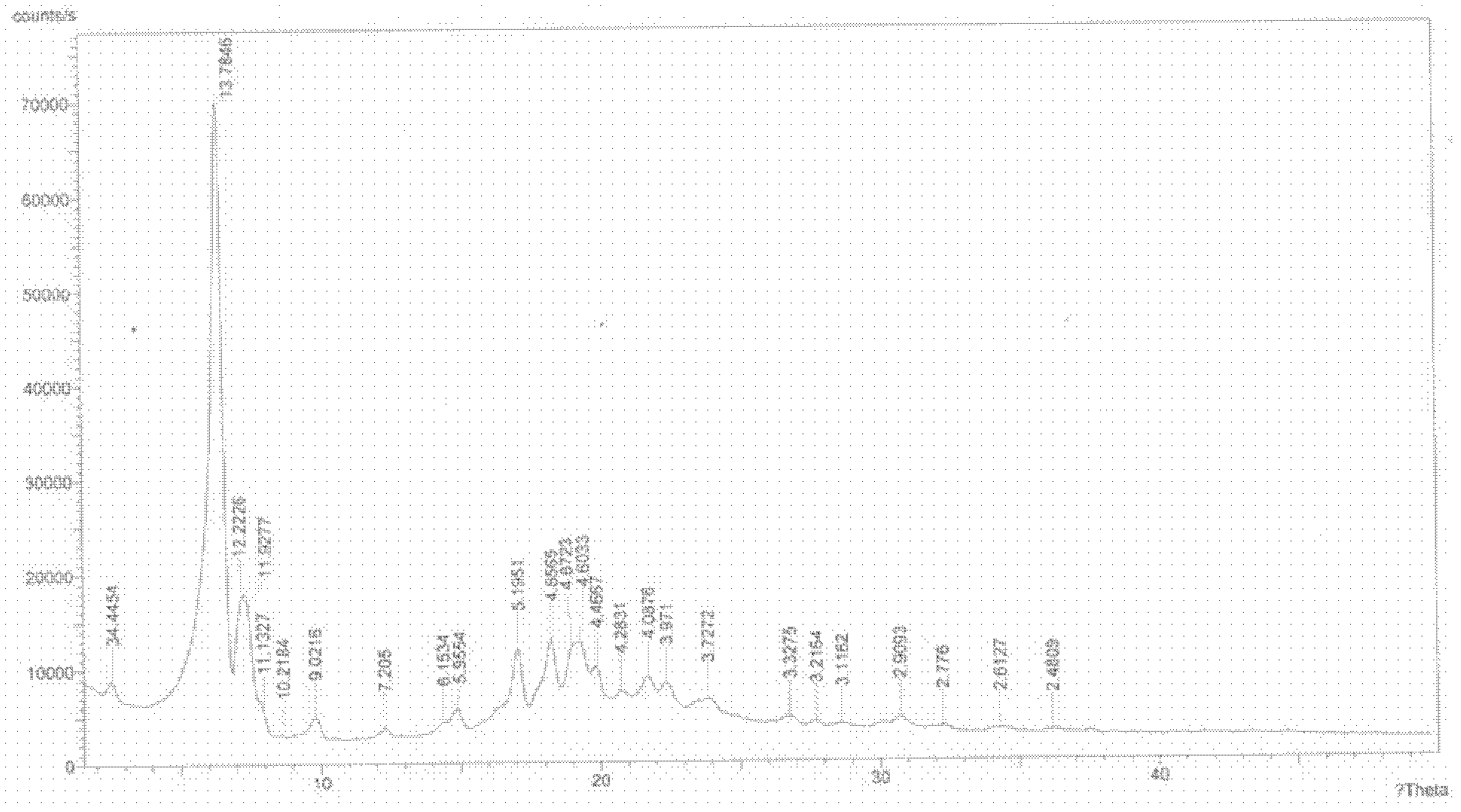Patents
Literature
54 results about "Injections pain" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
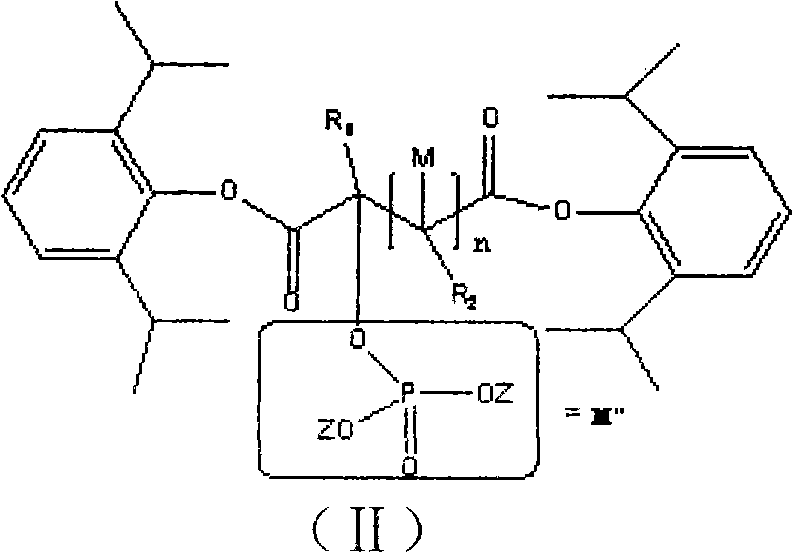
Phosphoryl carboxylic acid propofol ester derivative and preparation method thereof
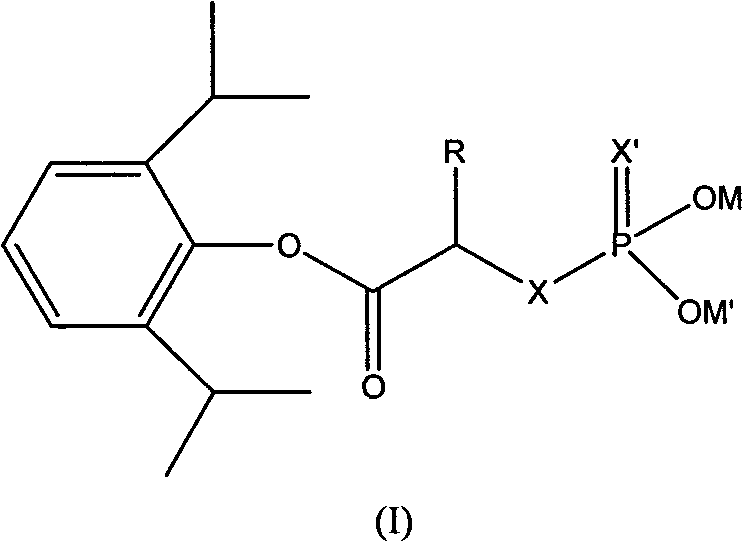
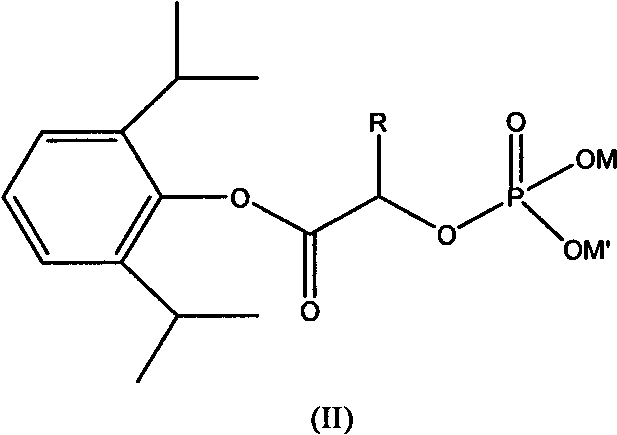
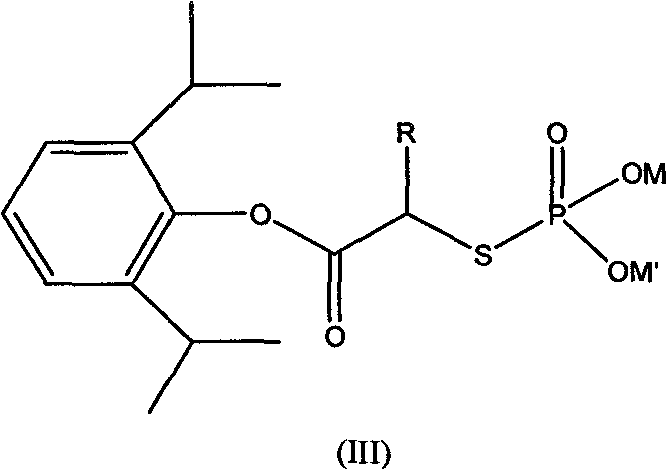
ActiveCN101633671AImprove bioavailabilityGood water solubilityOrganic active ingredientsAnaestheticsO-Phosphoric AcidPhosphate
The invention relates to a phosphoryl carboxylic acid propofol ester derivative which has the general formula (I). The method comprises the following steps: propofol reacts with 2-halogenated carboxylic acid and a derivative thereof by alkali to obtain corresponding ester and then the product reacts with phosphoric acid or thiophosphoric acid and the derivative thereof by dissolvent to obtain a water-soluble product or the propofol reacts with a 2-halogenated carboxylic acid phosphate ester derivative by the alkali to obtain the corresponding ester and then the ester is catalyzed, hydrogenated and salified to obtain the water-soluble product (I). The preparation method has mild reaction condition, high yield, simple operation and industrialized prospect, and a prepared oral preparation has the characteristics of high bioavailability, rapid absorption, high stability, and the like; and auxiliary materials with safety defects, such as a surface active agent, and the like can not be added into the prepared injection, thereby improving the stability of the preparation, reducing or removing injection pain, increasing the compliance of patients, overcoming the defects of propofol emulsion and having the advantage of obvious effect. The invention has the structural general formula (I).
Owner:HANGZHOU ADAMERCK PHARMLABS INC
Traditional Chinese medicinal lotion formula for treating exogenous fever
InactiveCN101987137AAnthropod material medical ingredientsHydroxy compound active ingredientsOral medicineDisease
The invention relates to a lotion formula which fulfills the aim of treating exogenous fever by traditional Chinese medicine washing therapy. The formula can achieve a satisfactory treatment effect on the exogenous fever by washing with a traditional Chinese medicine without injection or medicine taking, and solves the problem of injection pains and medicine-taking suffering, and particularly eliminates the harm of other oral medicines to human bodies. The traditional Chinese medicinal lotion is particularly suitable for infants who catch cold and have a fever, solves the problems of medicine-taking difficulty and injection pains of infants, and does not hurt tender entrails of the infants when treating diseases and abating fever.
Owner:梁婧卉
Active polypeptide capable of promoting osteogenesis and inhibiting osteoclast and application of active polypeptide
ActiveCN108276487AReduce osteoclastsReduce osteoporosisPeptide/protein ingredientsSkeletal disorderViewpointsCalcium phosphate coating
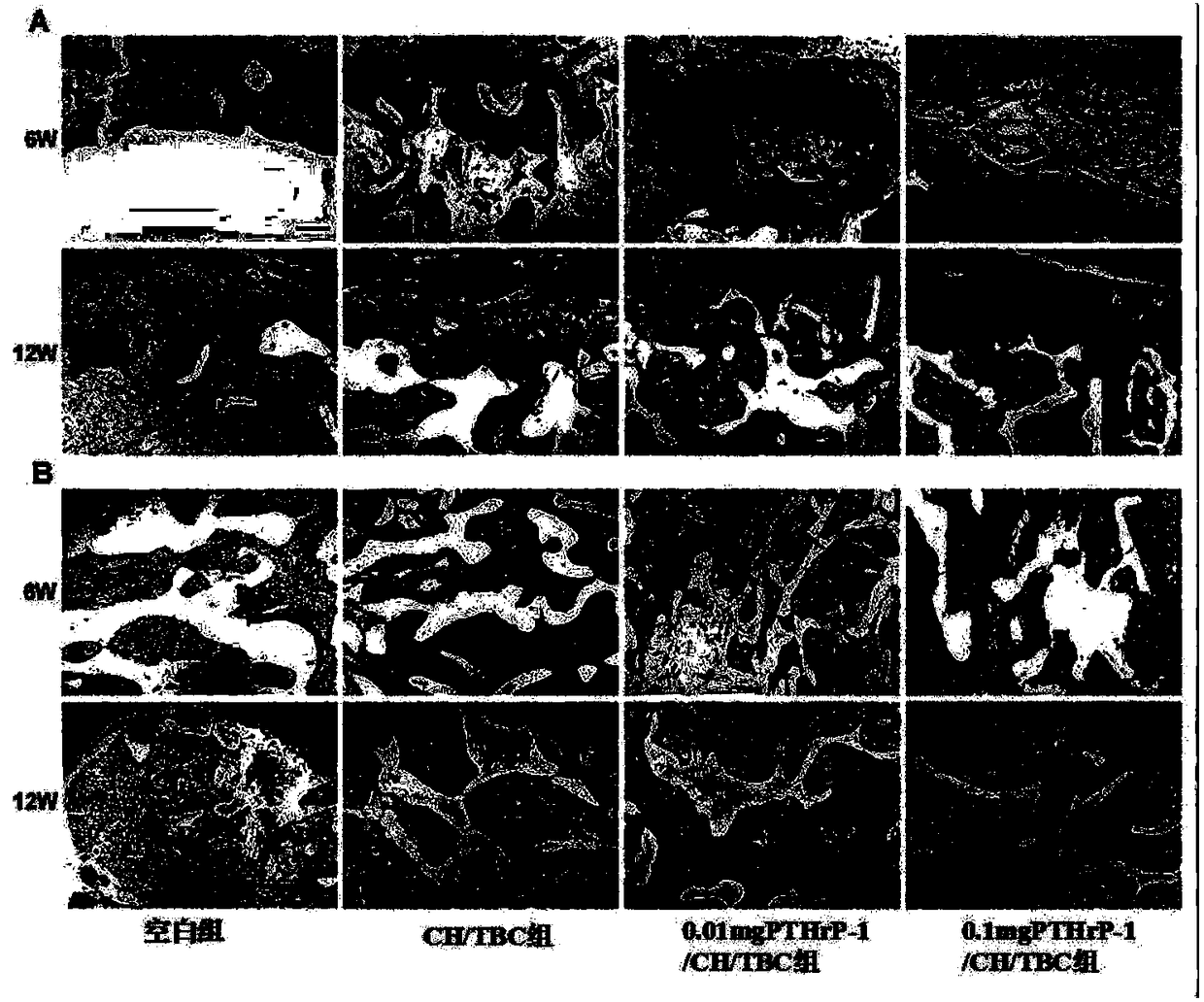
The invention discloses an active polypeptide capable of promoting osteogenesis and inhibiting osteoclast and application of the active polypeptide. N-terminal serine is phosphorylated on the basis ofPTH1-34, and three repeated sequences of glutamic acid or aspartic acid are introduced at a C terminal; the sequence of the polypeptide is shown in SEQ ID NO:1 or SEQ ID NO:2, and the polypeptide hasosteoinductive activity similar to BMP2 and an osteoclast inhibiting effect similar to parathyroid hormones (PTH). Random coils of the polypeptide can be avoided by bonding the C-terminal repeated sequences of the polypeptide to the surface of a calcium phosphate material or a material with a calcium phosphate coating, no addition of organic reagents is needed, and therefore the activity of the polypeptide is protected effectively; furthermore, slow controlled release can be achieved through cleavage of peptide bonds, and long-term intermittent injection of the PTH1-34 can be avoided, so thatthe injection pain of patients is reduced, and effects of osteoblast promotion and osteoporosis inhibition are achieved; a conventional viewpoint that the PTH1-34 cannot be administered locally is changed.
Owner:WUHAN UNIV
Albendazole nanoemulsion and preparation method thereof
InactiveCN102973505ADelay drug resistanceImprove stabilityOrganic active ingredientsAntiparasitic agentsSide effectFiltration
The invention belongs to the field of veterinary medicines, and discloses an albendazole nanoemulsion and a preparation method thereof. The albendazole nanoemulsion is prepared from the following raw materials by weight: 0.07-2.0% of albendazole, 31.8-38.3% of a surfactant, 0-12.7% of a cosurfactant, 0.54-9.9% of a solubilizer, 2.3-4.0% of oil, and 46.0-49.5% of water. The albendazole is completely dissolved in the solubilizer, and then the surfactant is added. The cosurfactant and the oil are mixed for using. Water is added to the solution prepared by the above steps at room temperature, while the solution is stirred constantly until the formation of a uniform and transparent system. The albendazole nanoemulsion is obtained. Compared with the prior art, the nanoemulsion of the present invention has the following advantages: 1) safety, efficiency and low drug resistance; 2) good stability, filtration sterilization, and easy storage; 3) rapid absorption, high bioavailability, targeted drug delivery, low side effects, small and uniform particle size, and good through-membrane absorption; 4) low-viscosity nanoemulsion, and little injection pain; and 5) easy use, and infinite dilution with water.
Owner:HENAN SOAR VETERINARY PHARMA
Injection powder and injection preparation of cefoperazone sodium-tazobactam combination
ActiveCN102552275AImprove efficacyAntibacterial agentsPowder deliveryCurative effectInjection powder
The invention relates to the technical field of medicine, in particular to an injection powder and injection preparation of a cefoperazone sodium-tazobactam combination. The injection powder and injection preparation comprises cefoperazone sodium, tazobactam combination and lignocaine hydrochloride, wherein the mass ratio of the cefoperazone sodium to the tazobactam combination to the lignocaine hydrochloride is 4:1:(0.01-0.05). Compared with a positive medicament control group, the injection powder and injection preparation of the cefoperazone sodium-tazobactam combination provided by the invention has the advantages of capability of relieving injection pain and remarkable improvement on the curative effect.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD +2
Temsirolimus for injection and preparation method thereof
ActiveCN103099806AAdvantages and Notable ImprovementsImprove stabilityOrganic active ingredientsAntineoplastic agentsAlcoholFreeze-drying
The invention belongs to the technical field of pharmaceutical preparations, and concretely relates to temsirolimus for injection and a preparation method thereof. The preparation method comprises dissolving a prescribed amount of temsirolimus and an anti-oxidant in anhydrous alcohol, mixing uniformly, adding a dispersant, re-mixing uniformly, freeze drying and removing the ethanol to obtain the temsirolimus. The preparation provided by the invention is few in prescription component; and the preparation method is convenient and easy, improves disadvantages of complex technology in present prescription, and greatly minimizes components such as alcohols and the like that may cause injection pain, thereby being relatively safe and reliable.
Owner:SHANDONG NEWTIME PHARMA
Propofol liposome freeze-drying preparation and preparation method thereof
ActiveCN105287406AInhibit aggregationIncrease the absolute value of Zeta potentialPowder deliveryHydroxy compound active ingredientsFat emulsionCholesterol
The invention discloses a propofol liposome freeze-drying preparation and its preparation method. The preparation is prepared from propofol, artificial synthetic phosphatide, cholesterol and a freeze-drying protectant. According to the preparation, artificial synthetic phosphatide is adopted to replace natural lecithin so as to avoid the hypersensitivity phenomenon which is easily caused by microprotein in natural lecithin. In addition, the preparation contains no auxiliary materials such as tween-80, EDTA-2Na, sulfite, thiosulfate and the like which are commonly used in a fat emulsion preparation, so as to further avoid the hypersensitivity phenomenon. The average particle size of the propofol liposome freeze-drying preparation is less than 100 nm such that injection pain caused by large particle size of a traditional propofol fat emulsion is avoided.
Owner:XIAN LIBANG ZHAOXIN BIOTECH CO LTD
Anesthetic nasal spray and preparation method thereof
InactiveCN109620802AEasy to acceptLow costOrganic active ingredientsAerosol deliveryPoor complianceSufentanil Citrate
The invention discloses an anesthetic nasal spray and a preparation method thereof. The anesthetic nasal spray can be used for overcoming local anesthesia injection pain in a minor operation of an adult outpatient clinic, can fully solve the problems of preoperative and intraoperative tension, poor compliance and poor sensitivity; the anesthetic nasal spray is prepared from, by weight, 3-5 ml of adexmedetomidine hydrochloride injection, 0.5-2 ml of a sufentanil citrate injection and 8-10 ml of normal saline. The preparation method of the anesthetic nasal spray comprises the following steps that a, the dexmedetomidine hydrochloride injection is sucked by a medical syringe A; b, the sufentanil citrate injection is sucked by a medical syringe B; c, the normal saline is sucked by the medicalsyringe B to dilute the sufentanil citrate injection; d, the liquids in the medical syringe A and medical syringe B are mixed, and then standing is performed for 3-10 minutes.
Owner:杜皓
Cefepime hydrochloride combined medicament
ActiveCN101822679APain reliefReduced pyrogenic reactionAntibacterial agentsOrganic active ingredientsCefepime hydrochlorideSuperficial phlebitis
The invention discloses a cefepime hydrochloride combined medicament, which removes medicament heat and anaphylactic reaction of cefepime hydrochloride and adverse reactions thereof on liver damage, injection pain, phlebitis produced by injection and the like in the prior art. The cefepime hydrochloride combined medicament has the advantages of safe use, stable quality and reliable curative effect. A preparation process for the medicament is energy-saving, environmentally-friendly and pollution-free.
Owner:邓学峰
Formula and preparation method of non-allergenic and painless novel propofol fatty microemulsion freeze-drying preparation
ActiveCN104523591AAvoid problemsAvoid oxidative degradationPowder deliveryHydroxy compound active ingredientsNatural sourceFreeze-drying
The invention discloses a formula and a preparation method of a non-allergenic and painless novel propofol fatty microemulsion freeze-drying preparation. The novel preparation comprises artificially synthesized phospholipid and artificially synthesized fatty acid glyceride, the presence of such allergic sources as a trace of protein in lecithin and soybean oil as natural sources is avoided, the uses of a metal complex EDTA, an antioxidant sulfite and thiosulfate is avoided, the allergic reaction caused by a metal complexing agent and sulfide is eliminated; a water phase free propofol is removed through various means in the formulation and preparation process, so that the injection pain is alleviated or eliminated and thus the safety of the preparation and the compliance of patients are improved.
Owner:XIAN LIBANG ZHAOXIN BIOTECH CO LTD
Leading-in device with permeation sensing function
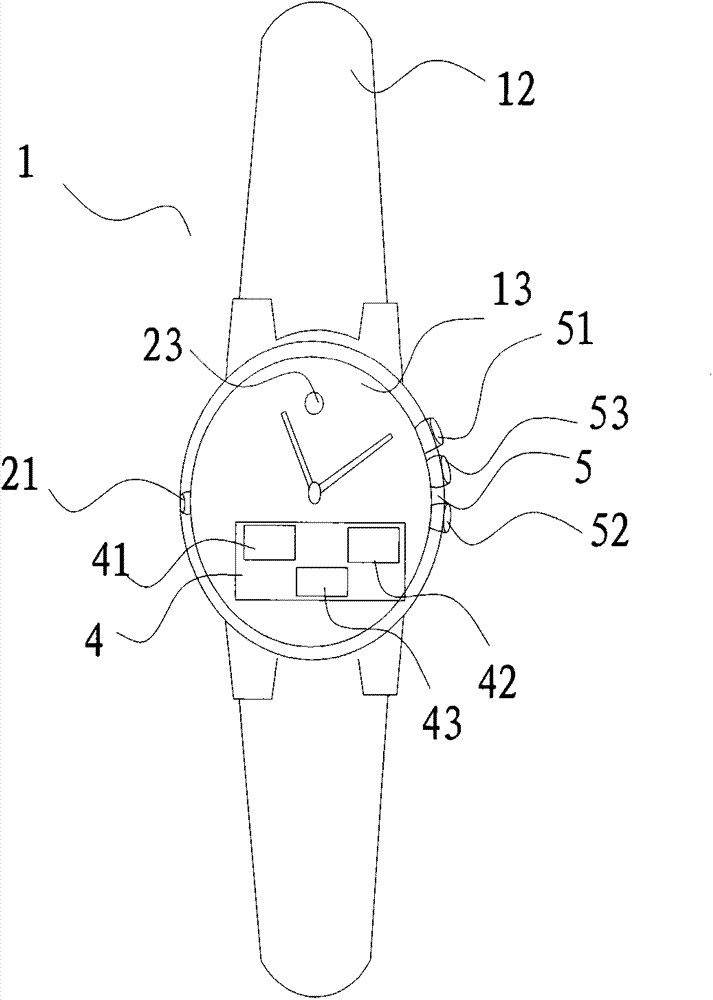
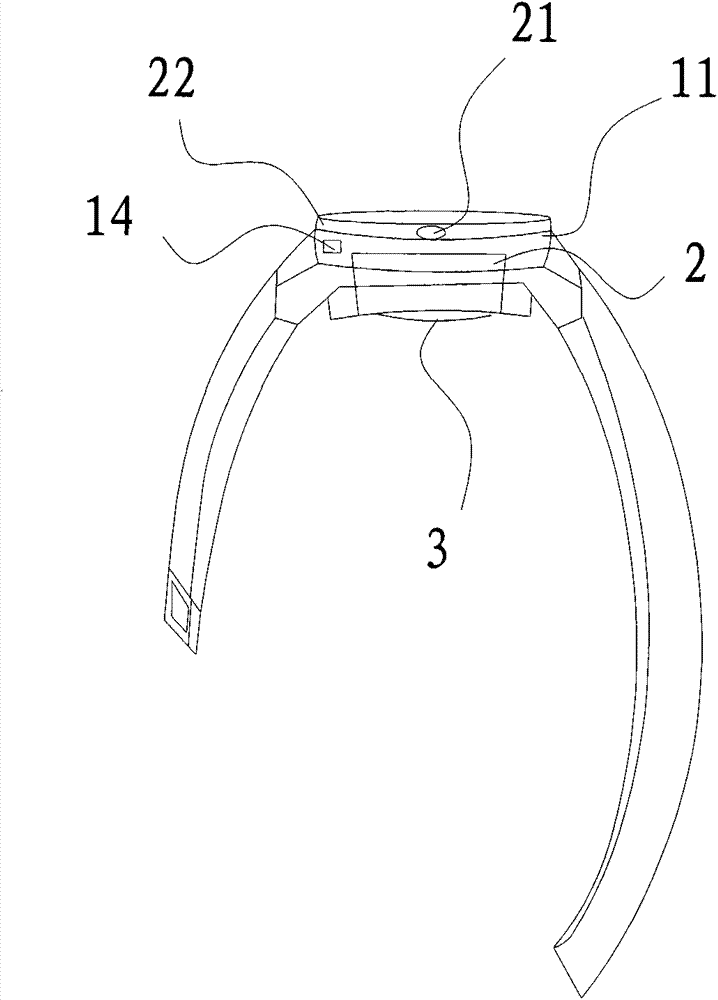
InactiveCN103768707AAvoid painSimple structureMedical devicesMechanical time indicationStart timeTime switch
The invention discloses a leading-in device with a permeation sensing function. The leading-in device with the permeation sensing function comprises a time device, a containing space, a gatherer device, a display structure and a control button. The time device is in the shape of a clock and comprises a base, a clock disk surface and a closk shell ring. The containing space is arranged in the time device and a user can inject insulin agentia into the containing space. The gatherer device is arranged at the position, making contact with the skin, of the base of the time device, and the insulin agentia enters the body through skin pores in a permeating mode. The display structure is arranged at the position of the clock disk face of the time device and the timing button control value, the leading-in time setting value and the injected insulin agentia amount are all displayed through the display structure. The control button is arranged at the position of the clock shell ring and the functions of prompting of the time when injection is needed, permeation time setting and starting time switch use are achieved. Thus, the clock and the insulin agentia can be combined, and therefore the problems of injection pain, injection forgetting and field limiting are solved.
Owner:陈宁飞
Propofol compounds
InactiveCN101224298ARelieve painSimple processHydroxy compound active ingredientsPeptide/protein ingredientsMedicineCyclodextrin
The invention belongs to the pharmaceutical field, which relates to a propofol combination. The weight proportion of the materials contained in the combination is: propofol: hydroxypropyl-B-cyclodextrin: albumin is equal to 0.1-2 : 1-35 : 1-35. The combination has good stability, simple production technique and low cost, and the injection pain is alleviated obviously. .
Owner:常州安孚立德药业技术有限公司
Propofol composition
InactiveCN101590029ARelieve painSimple processHydroxy compound active ingredientsAnaestheticsCyclodextrinAlbumin
The invention belongs to the pharmaceutical field, and relates to a propofol composition. The composition contains the following components in ratio by weight that propofol: cyclodextrin: albumin is 0.1-3:0.1-50:0.01-30. The composition has the advantages of good stability, significantly reduced injection pain, simple production process and low cost.
Owner:常州安孚立德药业技术有限公司
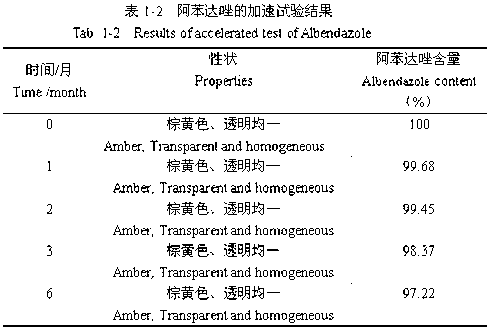
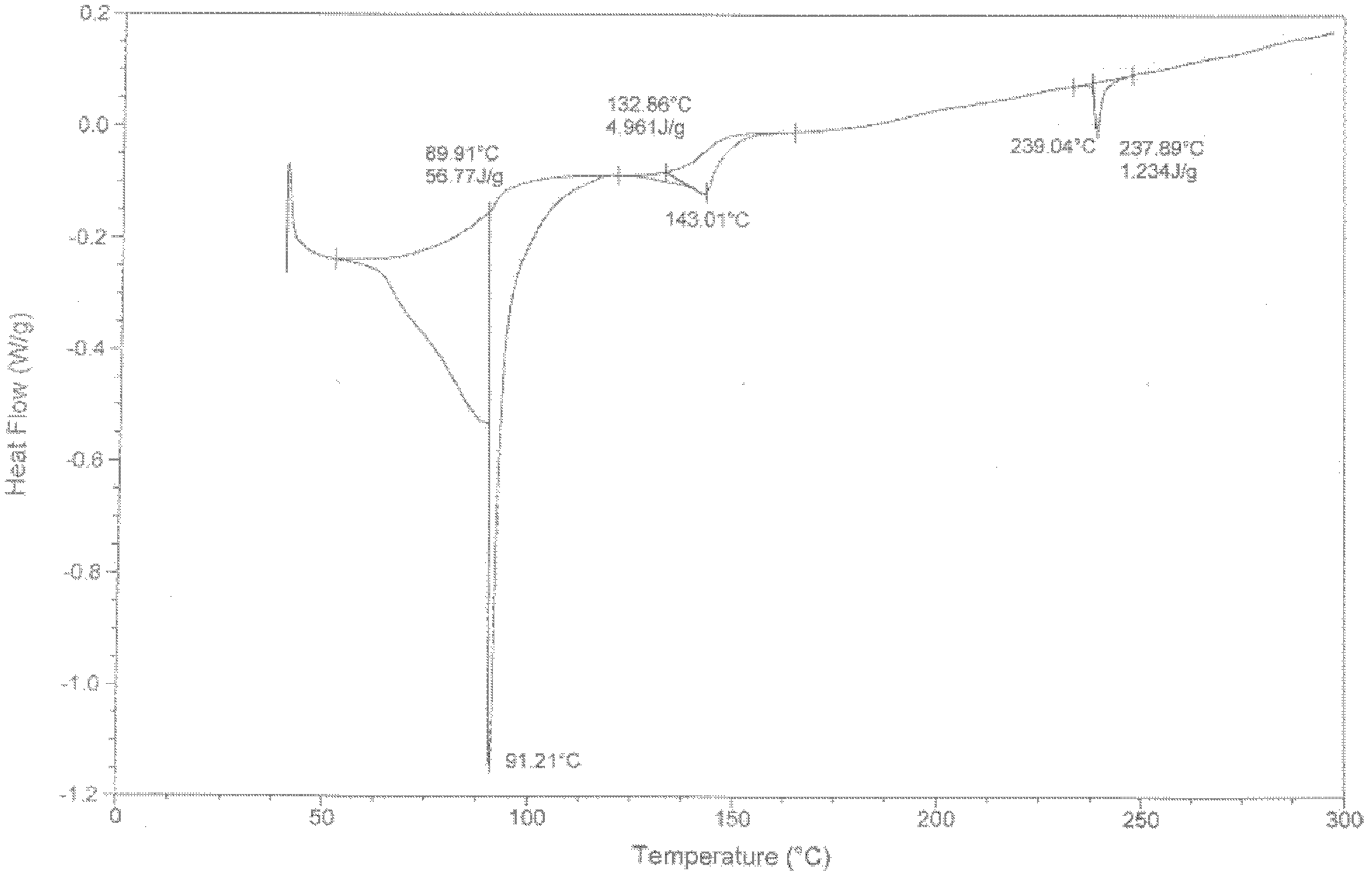
Sodium valproate crystal form as well as preparation method and application thereof
ActiveCN102603510AQuality improvementSignificant effectNervous disorderAnhydride/acid/halide active ingredientsX-rayCurative effect
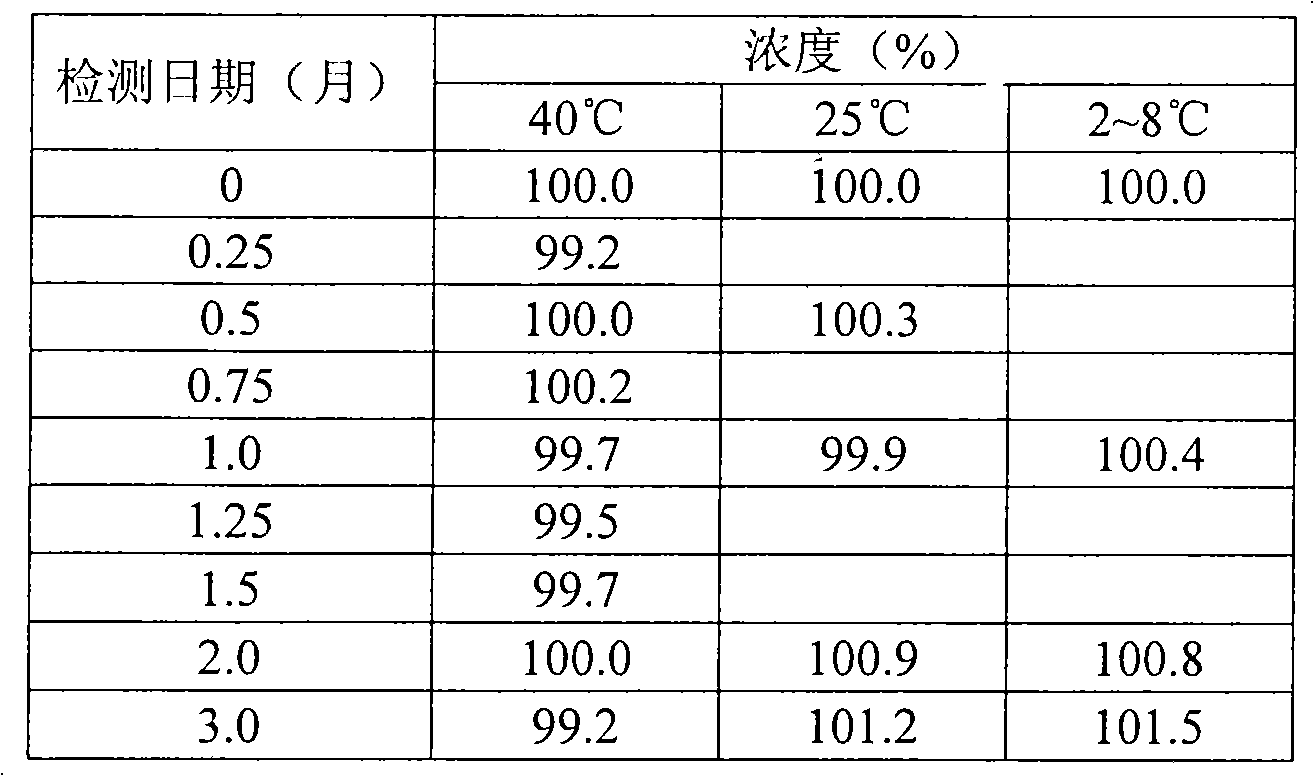
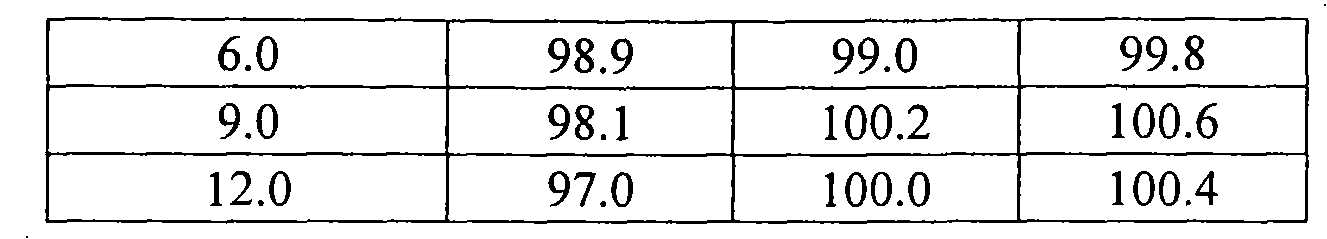
The invention discloses a sodium valproate crystal form II as well as a preparation method and application thereof, wherein characteristic absorption peaks appear at diffraction angles of (2theta)=6.3-6.5 DEG, 7.12-7.32 DEG, 7,3-7.5 DEG, 16.95-17.15 DEG, 18.15-18.35 DEG, 18.88-19.08 DEG and 19.17-19.37 DEG in a powder x-ray diffraction pattern of the crystal. The sodium valproate crystal form II provided by the invention has the advantages that the hygroscopicity is significantly reduced and lower water content of the product is guaranteed to ensure that the quality of the sodium valproate is more stable in storage period; while the hygroscopicity is reduced, the dissolution time of the product is significantly shortened and the dissolvability of the product is improved to ensure that the curative effect and very good safety of the sodium valproate are guaranteed in clinical application, the injection pain is also significantly relieved, compliance of patients is increased during treatment and very good clinical effect of the treatment is obtained.
Owner:SICHUAN CREDIT CHEMWERTH PHARMACEUTICAL CO LTD
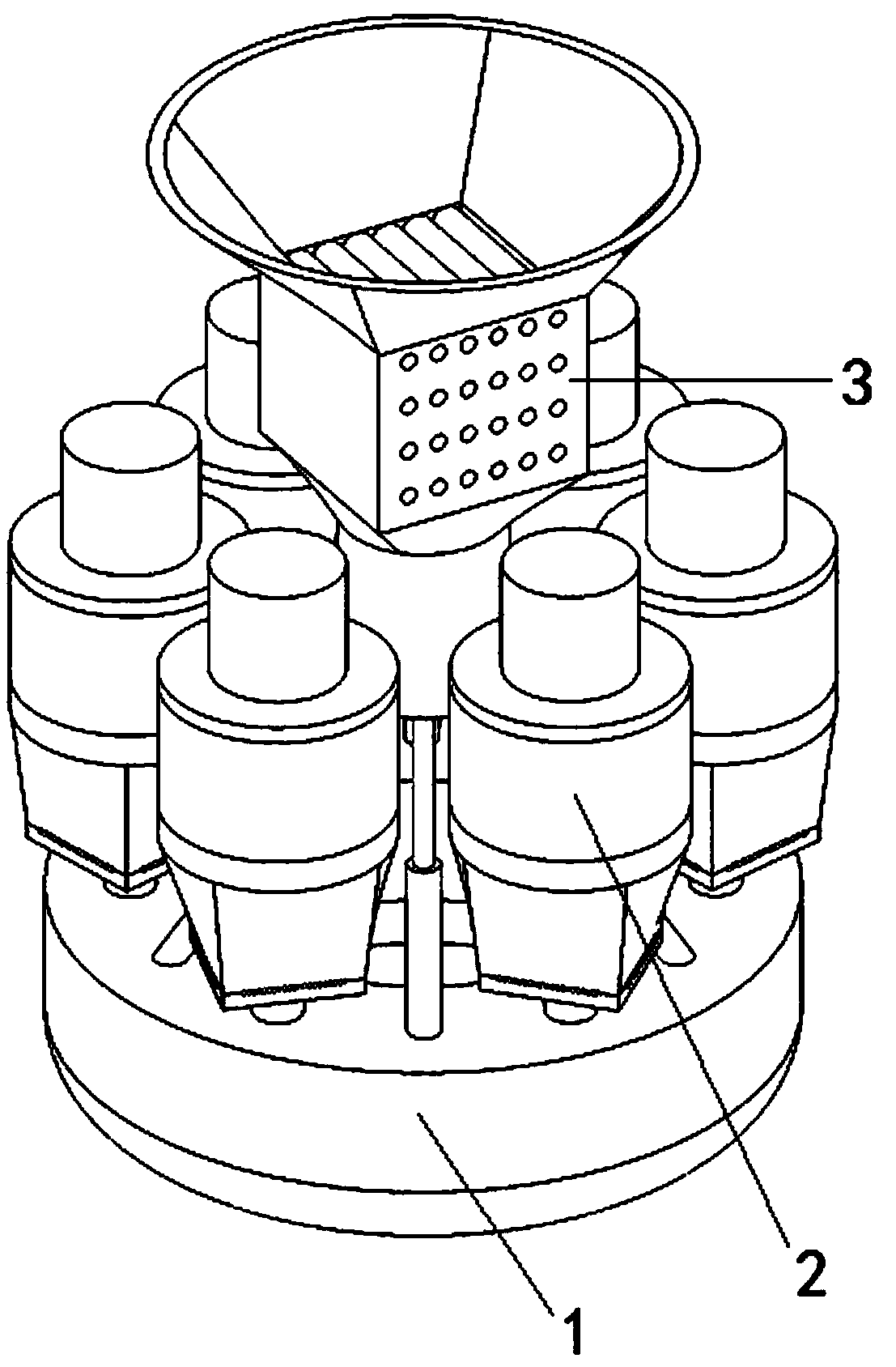
Pain-relieving medicine liquor for external application and preparation method and preparation device thereof
ActiveCN108969664AThe drug works quicklyGuaranteed qualityHydroxy compound active ingredientsAntipyreticExternal applicationSide effect
The invention discloses a pain-relieving medicine liquor for external application and a preparation method and a preparation device thereof. The preparation method comprises the following steps of selecting of materials, primary crushing, soaking, primary boiling, filtering, secondary crushing, secondary boiling, mixing again, and disinfecting. The preparation device comprises a mixing and boilingmechanism and six crushing mechanisms, wherein the crushing mechanisms are uniformly distributed at the edge of the top part of the mixing and boiling mechanism along the circumference direction; a stirring mechanism is vertically arranged at the center of the top part of the mixing and boiling mechanism. The pain-relieving medicine liquor has the advantages that the effect is quickly taken, andany toxic or side effect to the liver is avoided; the pain-relieving function is realized, the treatment function is also realized, the poor irritation to skin is avoided, the pain-relieving effect isobvious, and the pain-relieving function is obvious; compared with the oral pain-relieving medicines and injection pain-relieving medicines, the convenience in use is realized, the process of the preparation method is simple, and the good guarantee is provided for the quality of the pain-relieving medicine liquor; the preparation device can be combined with the preparation method, the use requirement of each step in the whole preparation method can be met, the preparation efficiency of the pain-relieving medicine liquor is greatly improved, and the safety and quality of the pain-relieving medicine liquor are guaranteed.
Owner:LUOHE MEDICAL COLLEGE
Medicinal composition using anterior pituitary adrenal cortical extract as main component, and its preparation method and use
A composite medicine for treating arthritis and viral cold and preventing cancer and injection pain contains corticotropic hormone, thymopeptide and immune ribonucleic acid, which are extracted from anterior pituitary adrenal cortex, and the process for preparing its liquid (or freeze-dried powder) injection are disclosed.
Owner:蔡海德
Syringe for painless oral local anesthesia
The invention relates to a pain-preventing syringe, in particular to a syringe for painless oral local anesthesia, and solves the problems that the traditional local anesthesia by manual injection causes intense piercing pain and injection pain and makes a patient to be in fear of injection, feel anxiety, be unwilling for treatment, and even give up the treatment mentally. The syringe comprises a needle, a tube, a quick connector, a battery, a shell, a motor, a reducer, a coupling, a screw, a screw nut, and a push rod. One end of the needle is connected with one end of the tube, and the other end of the tube is connected with one end of the shell. The battery is disposed inside the other end of the shell and is connected with the motor. A motor rotary shaft is connected with the reducer which is connected with one end of the screw through the coupling, and the other end of the screw passes through the screw nut to be connected with one end of the push rod. The other end of the push rod is connected with a piston rod at the other end of the tube. The screw nut is fixedly mounted in the shell. The syringe is applicable to the field of medical science.
Owner:保占波
Cough relieving cigarette
InactiveCN101366739AAchieve the effect of relieving cough and removing phlegmSolve bad shortcomingsPharmaceutical delivery mechanismRespiratory disorderMedicinal herbsSide effect
The invention relates to a cough-relieving expectorant medical cigarette, which is totally made from Chinese medicinal herbs. The clinical tests of thousands of patients in six years verify that the medical cigarette has magical efficacy to acute and chronic bronchitis, and no adverse reaction is observed. The product is designed into a long tube shape, which is convenient to carry. Six cigarettes form one course, which is more convenient to use. Meanwhile, the product initiates a sucking method for relieving cough, has no side effect on gastrointestinal tract, has no injection pain, and is effective on a plurality of types of cough and asthma as well. According to statistics, the effective rate of the product for a plurality of types of cough reaches 84 percent.
Owner:王治中 +1
Novel painless diluent, dilution compatibility method and application of alprostadil fat emulsion preparation
The invention discloses a painless alprostadil fat emulsion composition and a preparation method thereof. The fat emulsion composition is prepared from alprostadil fat emulsion and blank fat emulsion according to the minimum ratio of 1:5. By taking active adsorption, physical isolation and protection of the stability of the preparation as strategies, a novel preparation diluting method is established, injection stimulation and injection pain caused by the alprostadil fat emulsion preparation can be removed or obviously reduced, and the treatment tolerance of a patient is improved.
Owner:XIAN LIBANG PHARMA
Traditional Chinese medicine for treating children cough and preparation method of external use patch thereof
InactiveCN103006986AGood treatment effectSolve the problem of protracted diseaseRespiratory disorderSheet deliveryMoisture absorptionAngelica dahurica
The invention discloses a traditional Chinese medicine for treating children cough. The traditional Chinese medicine is composed of the following components in weight percent: 5.88-37.34% of forsythia, 2.34-29.43% of angelica dahurica, 2.34-29.43% of biond magnolia flower, 5.67-25.43% of Japanese yam, 5.67-28.43% of Chinese fevervine, 2.67-25.43% of balloonflower, 3.34-25.43% of aster, 5.67-25.43% of ladybell root, and 5.82-35.22% of gynostemma, wherein the sum of the weight percents of all components is 100%. The preparation method of external use patch thereof is as follows: firstly immersing, distilling, extracting and concentrating all components to obtain an extract including a distillate and an extractum, then mixing with pharmaceutic adjuvants, and finally obtaining the patch through applying, cutting, standing, cross bonding, secondary moisture absorption and quality inspection and package. The medicine and the patch have the effects of dispelling wind, clearing heat, eliminating phlegm, stopping cough, dredging collaterals, inducing resuscitation, nourishing Yin and promoting body fluid, treat the children cough by the functions of antisepsis, antiviral, antipyretic, anti-inflammatory, eliminating phlegm, relieving cough, promoting digestion and improving body immunity, and meet the psychological characteristics of children during taking medicines, such as fear of bad taste and injection pain.
Owner:XIAN MEDICAL UNIV
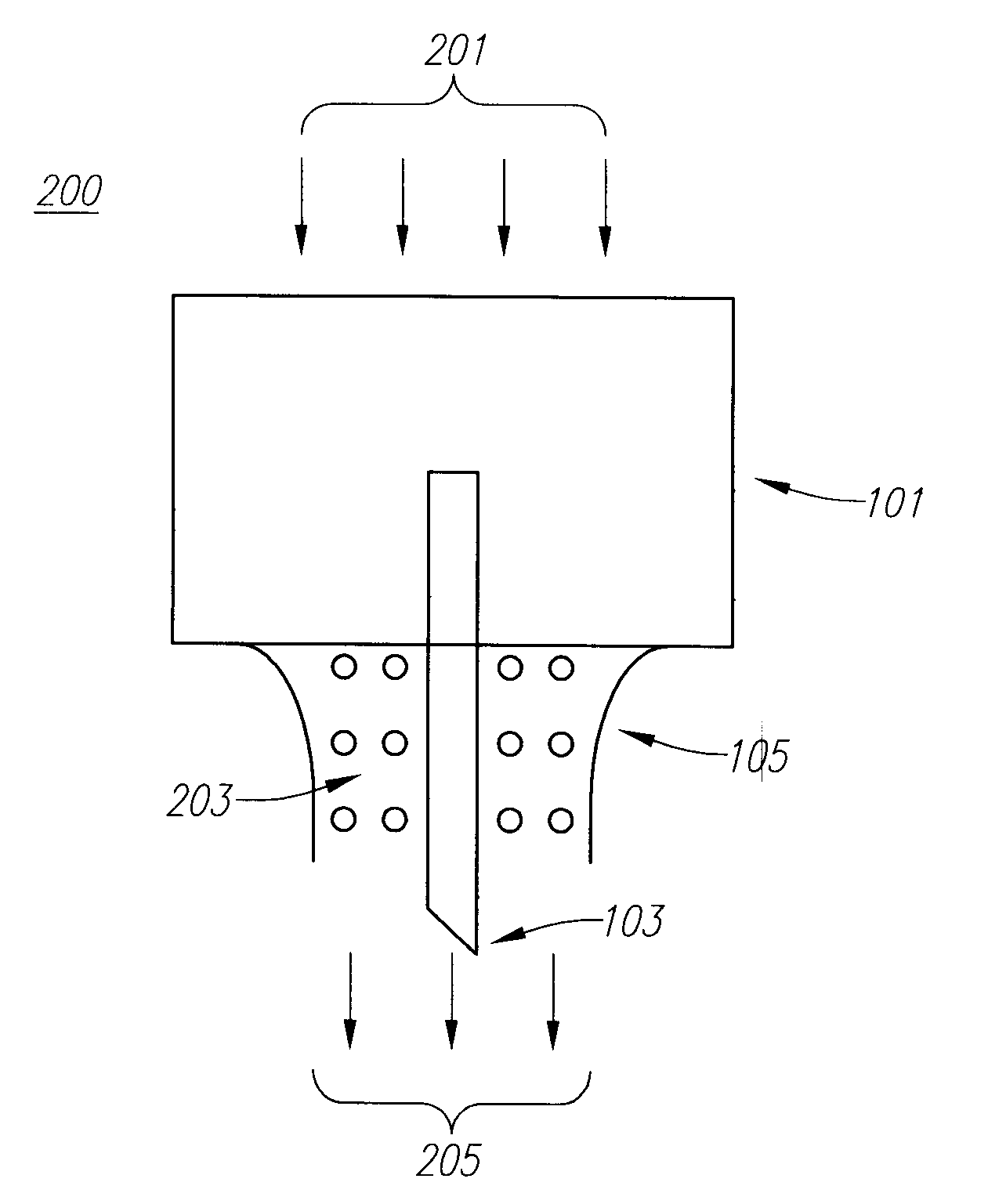
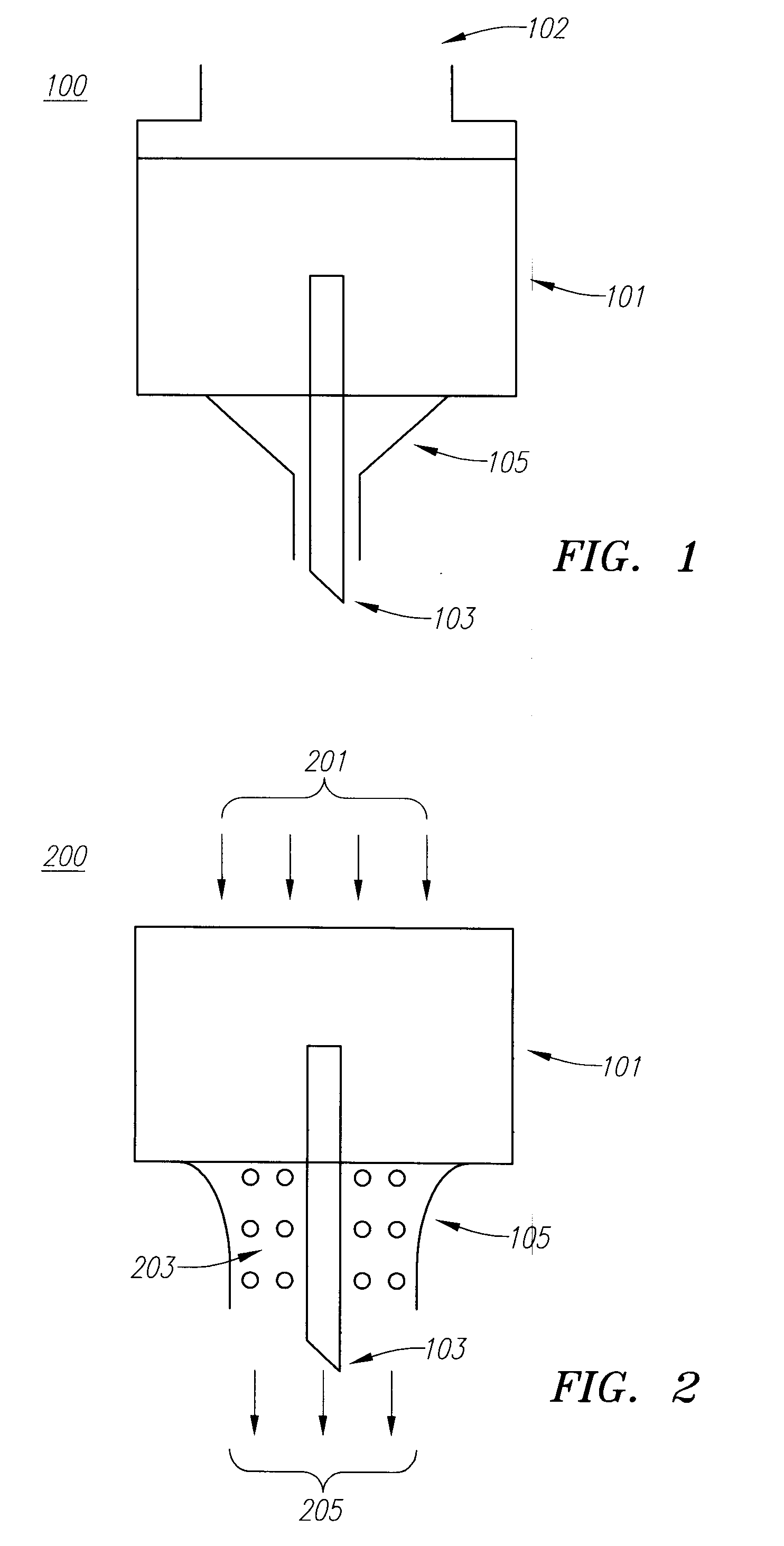
Drug Delivery Device
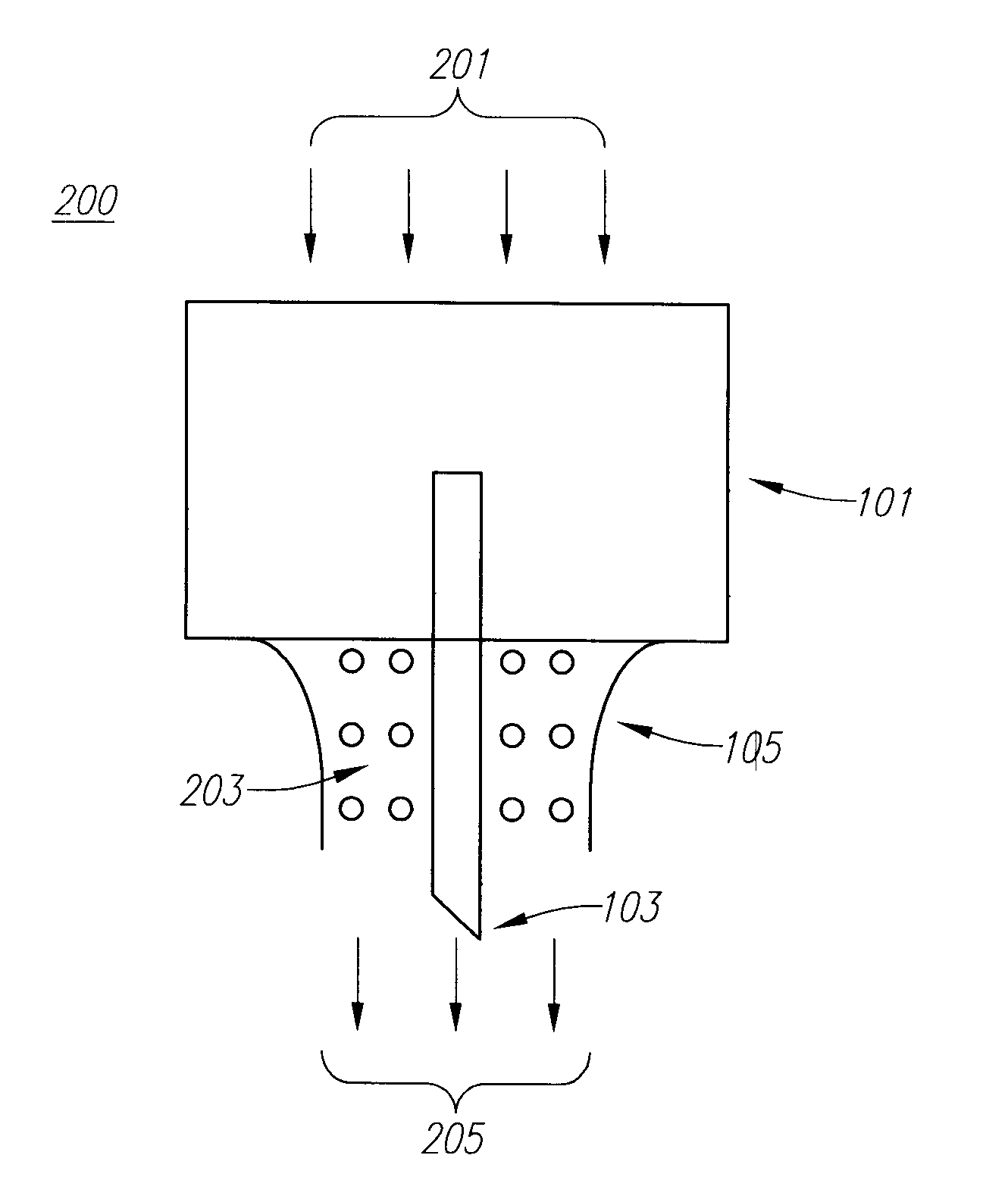
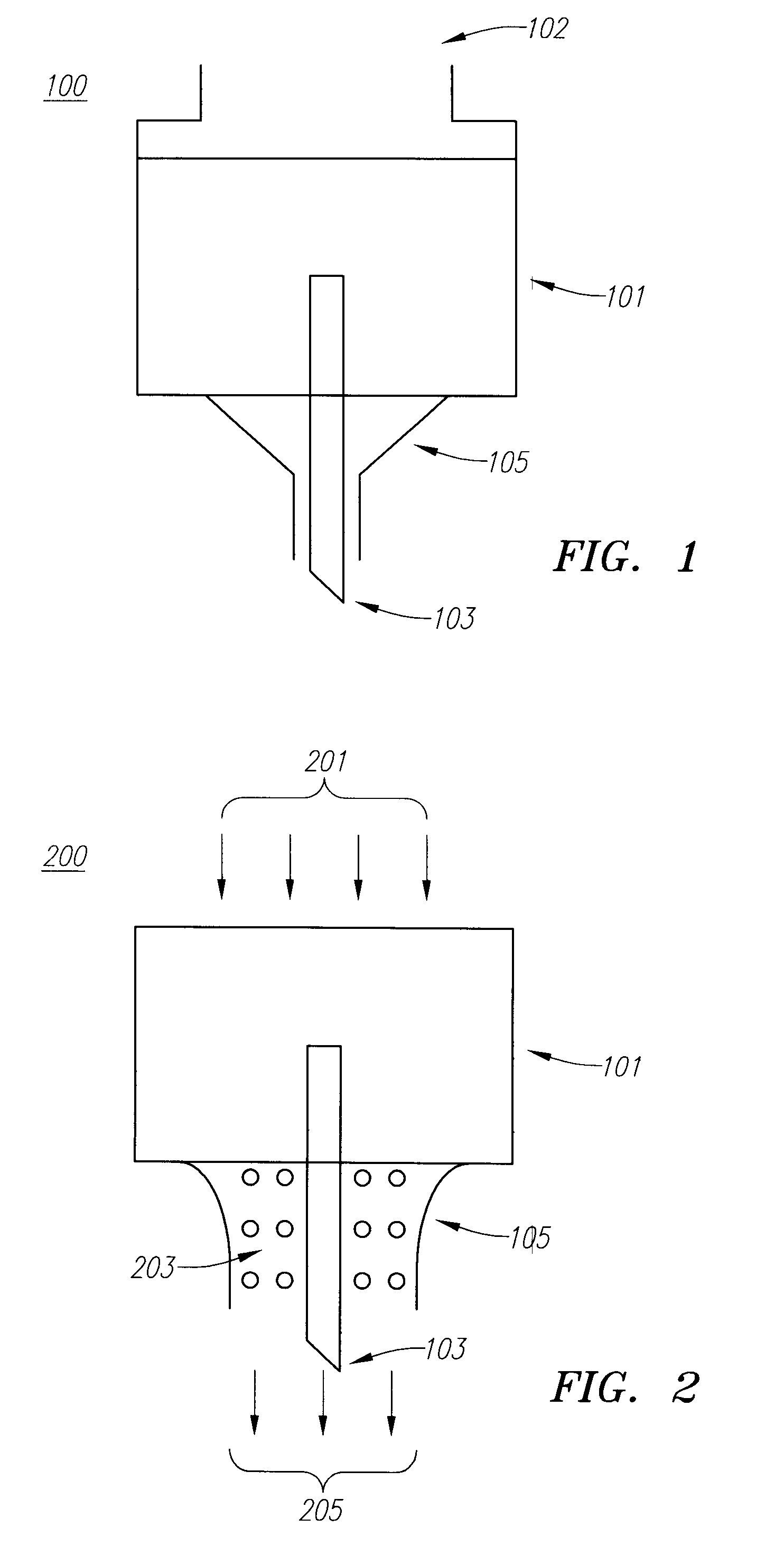
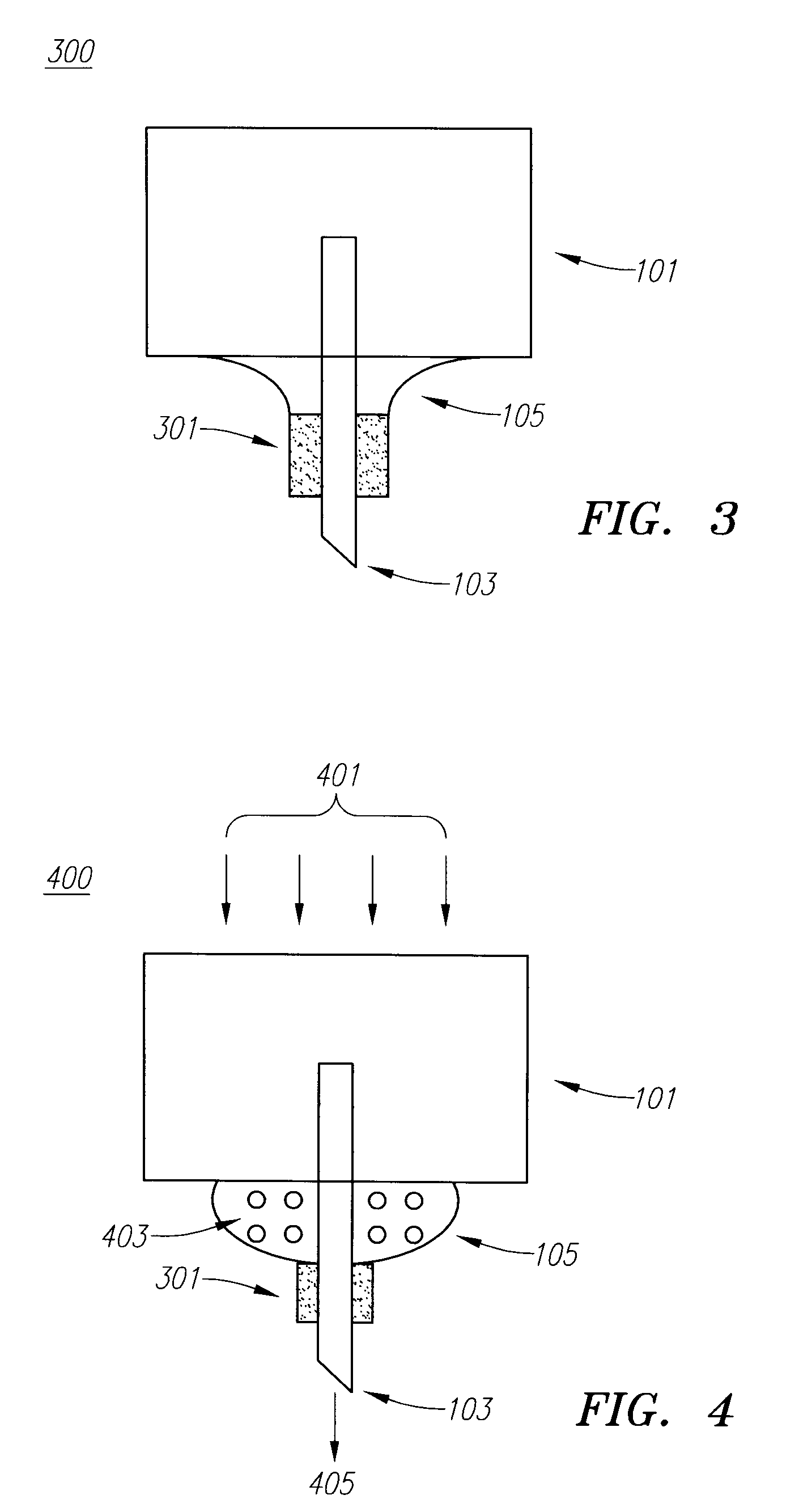
Embodiments of the invention generally provide a device and method for minimizing injection pain and preventing needle clogging during injection of a drug formulation into skin. Generally, the invention provides a device comprising a needle having a point to penetrate skin on one end, a hub having a diameter larger than that of the needle attached to the opposite end of the needle and connectable to a housing defining a chamber for receiving a formulation, and a polymer wrap attached to the hub, wherein the polymer wrap is tapered to the needle so as to pass through skin when the point is inserted into the skin, and wherein a space between the needle and the polymer wrap lies in a flow path of the formulation into the skin, such that formulation is injected through the space between the needle and the polymer wrap.
Owner:ASTRAZENECA PHARMA LP
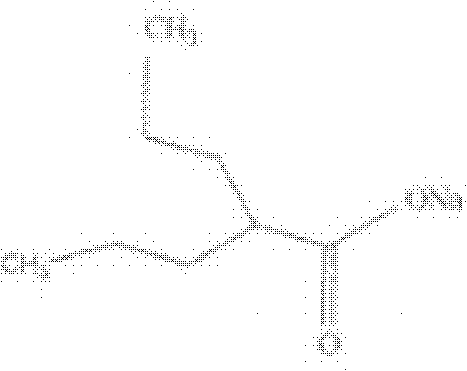
Water-soluble dipropofol and preparation method thereof
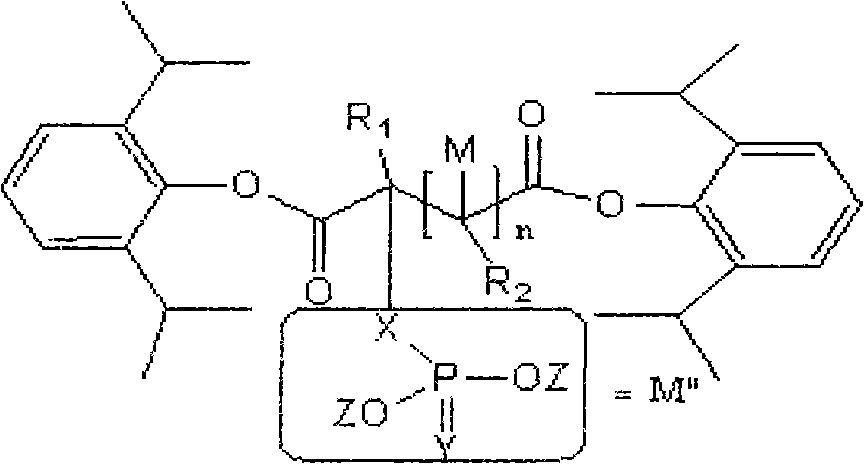
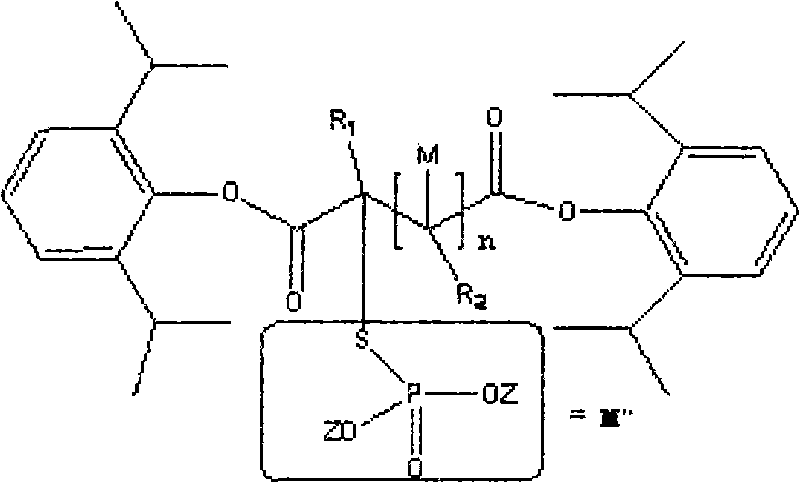
InactiveCN101863918AGood water solubilityStrong anesthetic effectAnaestheticsPhosphorus organic compoundsSolubilityInjections pain
The invention discloses water-soluble dipropofol and a preparation method thereof. The water-soluble dipropofol has a certain general formula structure. The preparation method comprises the following steps of: reacting propofol, dihalogen acid and derivative thereof to generate diester under the alkali condition, and reacting the diester, phosphoric acid, thiophosphoric acid and derivatives thereof to obtain the water-soluble dipropofol. By introducing two-molecule propofol, the water-soluble dipropofol reduces the intake of the phosphorus, and has quick response; and the preparation method has the advantages of easily obtained raw materials, mild reaction conditions, convenient operation and easy industrialized production. The prepared injection has good water solubility and high stability, and enlarges the using crowds of the medicament. The water-soluble dipropofol can overcome the defects that the propofol emulsion is instable on thermodynamics and dynamics, easy to pollute and propagate microbes and inconvenient to store and use, can reduce or remove injection pain, reduces the intake of the phosphorus, and has quick response.
Owner:李世系 +1
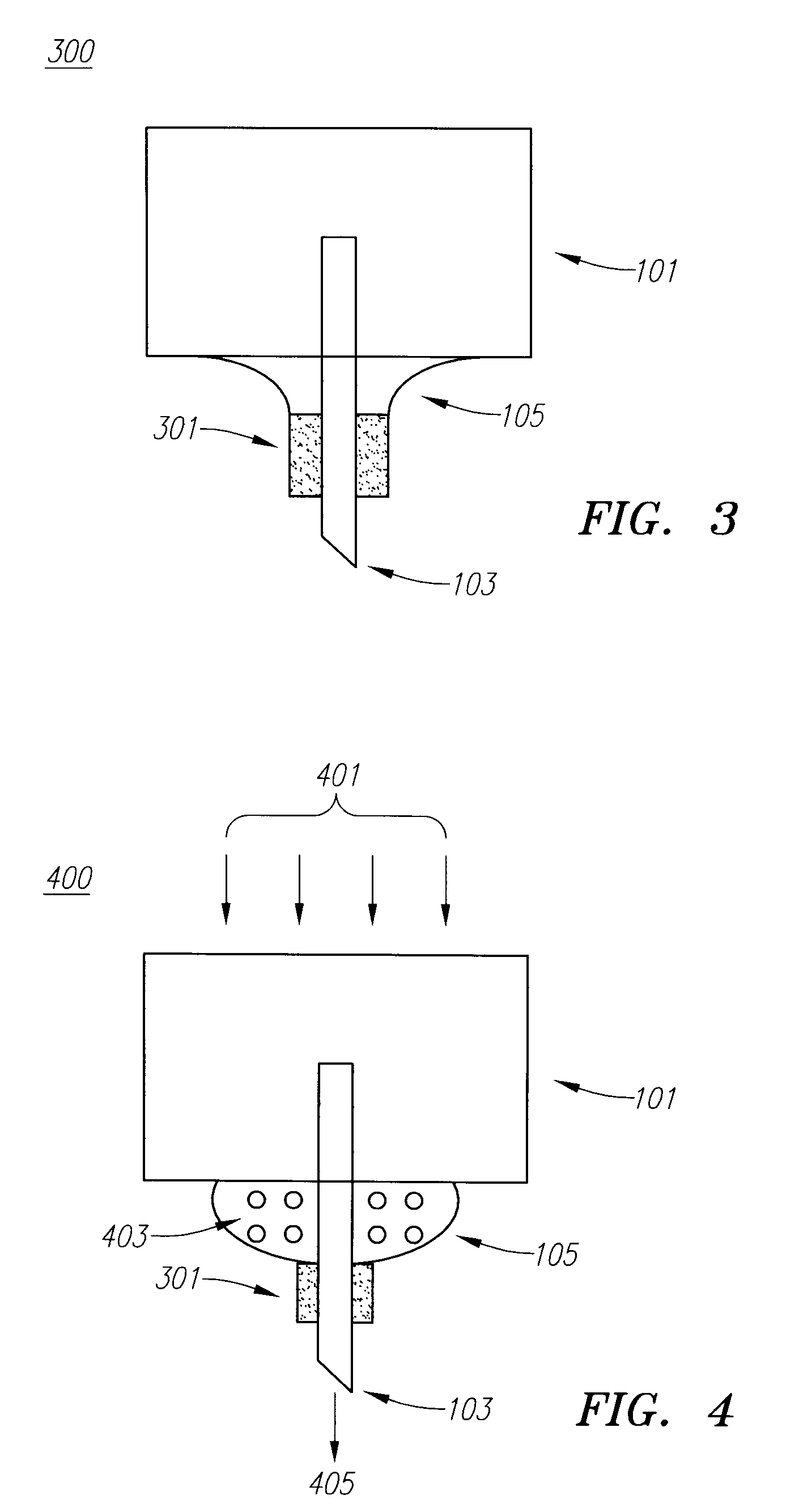
Drug delivery device
Embodiments of the invention generally provide a device and method for minimizing injection pain and preventing needle clogging during injection of a drug formulation into skin. Generally, the invention provides a device comprising a needle having a point to penetrate skin on one end, a hub having a diameter larger than that of the needle attached to the opposite end of the needle and connectable to a housing defining a chamber for receiving a formulation, and a polymer wrap attached to the hub, wherein the polymer wrap is tapered to the needle so as to pass through skin when the point is inserted into the skin, and wherein a space between the needle and the polymer wrap lies in a flow path of the formulation into the skin, such that formulation is injected through the space between the needle and the polymer wrap.
Owner:ASTRAZENECA PHARMA LP
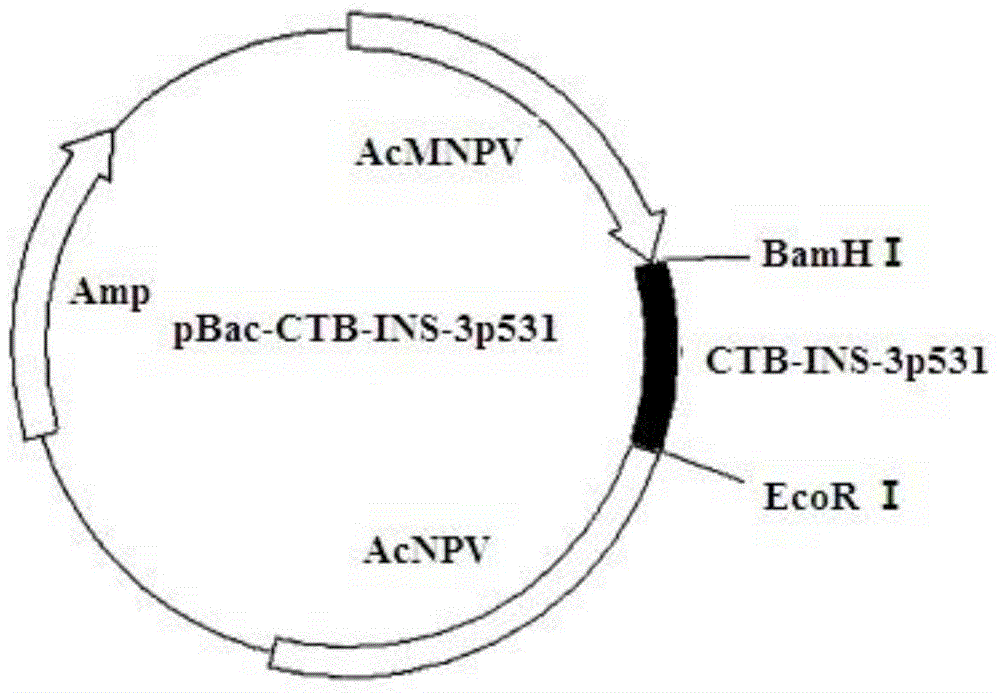
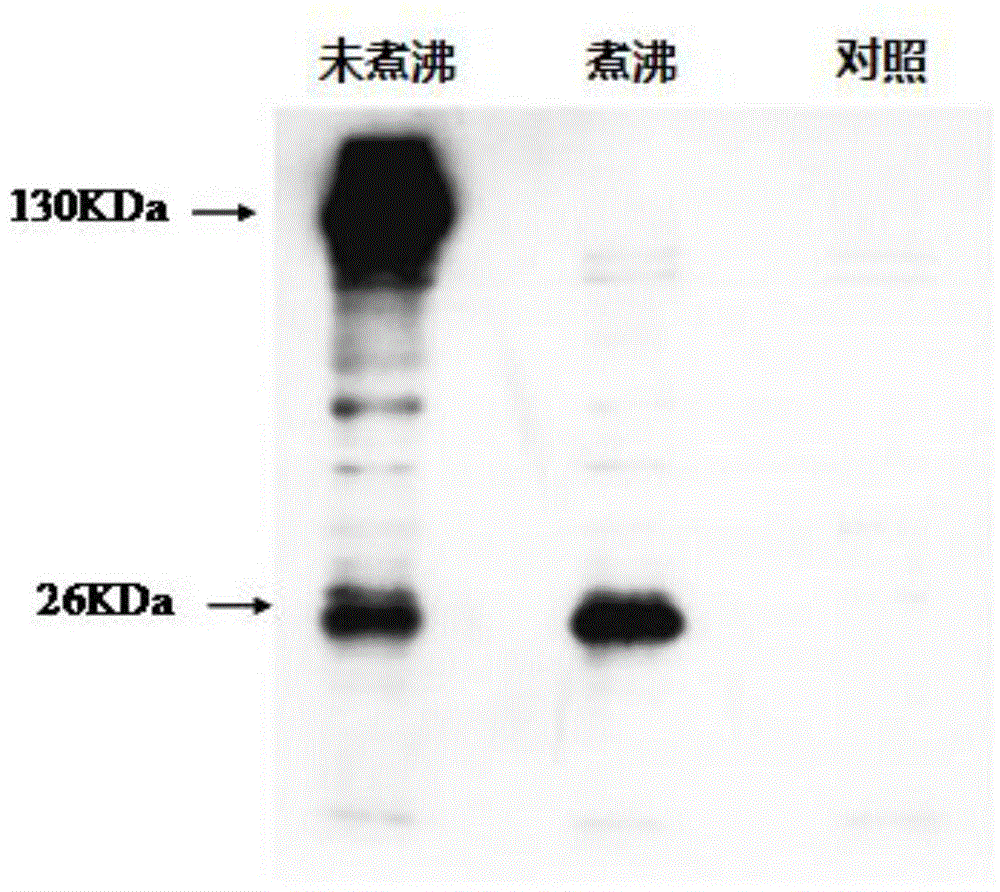
Fusion protein of CTB (Cellulose Tribenzoate), human insulin and glutamic acid decarboxylase 3p531 fragments and application thereof
ActiveCN103820477ARelieve painEliminate the threat of infectionPeptide/protein ingredientsMetabolism disorderCelluloseHigh activity
The invention discloses a fusion protein of CTB (Cellulose Tribenzoate), human insulin and glutamic acid decarboxylase 3p531 fragments and application of the fusion protein. The application of the fusion protein is in preparing a medicament for treating type I diabetes. Silkworm larvae or silkworm chrysalides infected by recombinant silkworm baculoviruses efficiently express the fusion protein which has higher activity than that of the fusion protein expressed by prokaryotes directly and higher yield than that of the fusion protein expressed by transgenic plants; a silkworm body has multiple natural protein protective agents, thereby having a protection function for expression products and facilitating the more stable expression products; the prepared fusion protein can be prepared into the medicament for treating the type I diabetes and relieving injection pains and infection threats for patients.
Owner:ZHEJIANG UNIV
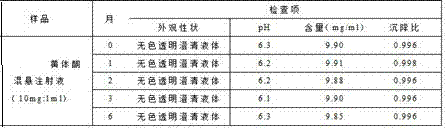
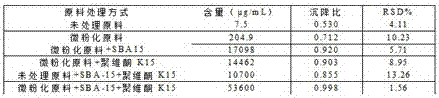
Progesterone suspending injection and preparation method thereof
InactiveCN107441039AGood dispersionGood suspensionOrganic active ingredientsSolution deliveryCrystal structureProgesterones
The invention relates to progesterone suspending injection and a preparation method thereof. The progesterone suspending injection comprises progesterone, SBA-15, povidone K15 and injection water. According to the invention, under the effect of physical mechanical force, the well-organized crystal structure of the progesterone is damaged but is not suffered from chemical degradation and SBA-15 and progesterone molecule are interacted through hydrogen bond, so that the amorphous progesterone molecule is loaded onto a nanometer structure duct, can be attached to the SBA-15 loading a large quantity of drug molecules and can be maintained under amorphous state; the amorphous progesterone molecule reacts with the povidone K15 through the hydrogen bond, so that the amorphous state is more stable and a certain suspending assisting function is achieved; the technical problems that the progesterone is difficult to dissolve in water, the oil-soluble progesterone injection brings a strong injection pain response and a conventional solubilizing method is not suitable for preparing the progesterone into the injection can be solved; and the progesterone is developed into the injection with stable nature and without pain response in the injection process.
Owner:南京斯泰尔医药科技有限公司
Injector for children
The invention belongs to injectors, and particularly relates to an injector for children. The injector for children relieves injection pain conveniently and comprises an outer shell, wherein an arc opening is formed in the bottom of the outer shell, a needle head hole is formed in the middle of the arc opening, a needle tube is arranged in the middle of the outer shell, the bottom of the needle tube is connected with the needle head hole, the needle head hole is connected with a needle head array, a medicine-absorbing tube is arranged inside the needle tube, the needle tube is provided with degree scales, a rubber plug is arranged at the bottom of the needle tube, the rubber plug is in clearance fit with the needle tube, and the top of the rubber plug is connected with an air-blowing device. The injector for children likes a toy in appearance, can distract the attention of children, can relieve the resentment of children, and can improve the work efficiency of medical staff. After needle head are dismounted from the medicine-absorbing tube, the injector for children can serve as a toy and is interesting. More importantly, the injector for children is simple and accurate in injection operation and is more suitable for being used in households compared with a traditional injector.
Owner:WUXI X RES PROD DESIGN & RES
Propofol composition
InactiveCN101590029BRelieve painSimple processHydroxy compound active ingredientsAnaestheticsCyclodextrinAlbumin
The invention belongs to the pharmaceutical field, and relates to a propofol composition. The composition contains the following components in ratio by weight that propofol: cyclodextrin: albumin is 0.1-3:0.1-50:0.01-30. The composition has the advantages of good stability, significantly reduced injection pain, simple production process and low cost.
Owner:常州安孚立德药业技术有限公司
Cefamandole nafate combined drug
ActiveCN101822678APain reliefReduced pyrogenic reactionOrganic active ingredientsAntiviralsCurative effectSuperficial phlebitis
The invention provides a cefamandole nafate combined drug, overcoming cefamandole nafate fever reaction, anaphylactic reaction, harm to liver, injection pain, phlebitis caused by injection and other untoward reactions in the prior art. The cefamandole nafate combined drug is safe to use, stable in quality and reliable in curative effect. The preparation process is energy-saving, environment-friendly and pollution-free.
Owner:邓学峰
A kind of vecuronium bromide freeze-dried preparation and preparation method thereof
ActiveCN101843593BEffective and adequateImprove solubilityPowder deliveryOrganic active ingredientsSide effectFreeze-drying
The invention discloses a novel vecuronium bromide freeze-dried preparation and a preparation method thereof. The novel preparation comprises the main components of vecuronium bromide, a freeze-dried excipient, polyethylene glycol playing a role in re-dissolving assistance, vitamin E playing a role in anti-oxidation, and glycerol serving as an isotonic regulator. According to the formula, the vecuronium bromide is developed into the novel freeze-dried preparation, and the process is simple. The novel vecuronium bromide freeze-dried preparation can improve the pH value of re-dissolved preparation solution to make the pH value reach or be close to the physiological pH range of blood, avoids or reduces stimulations caused by the preparation to a blood vessel, alleviates injection pains, and simultaneously can ensure that a medicament compound is fully dissolved to prevent insoluble substances from causing side effect injuries to a body.
Owner:XIAN LIBANG PHARMA
Injection powder and injection preparation of cefoperazone sodium-tazobactam combination
ActiveCN102552275BImprove efficacyAntibacterial agentsPowder deliveryCurative effectInjection powder
The invention relates to the technical field of medicine, in particular to an injection powder and injection preparation of a cefoperazone sodium-tazobactam combination. The injection powder and injection preparation comprises cefoperazone sodium, tazobactam combination and lignocaine hydrochloride, wherein the mass ratio of the cefoperazone sodium to the tazobactam combination to the lignocaine hydrochloride is 4:1:(0.01-0.05). Compared with a positive medicament control group, the injection powder and injection preparation of the cefoperazone sodium-tazobactam combination provided by the invention has the advantages of capability of relieving injection pain and remarkable improvement on the curative effect.
Owner:SHANDONG LUOXIN PHARMA GRP CO LTD +2
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com