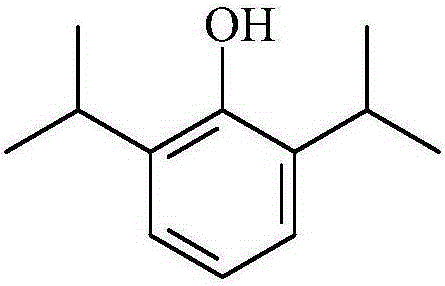
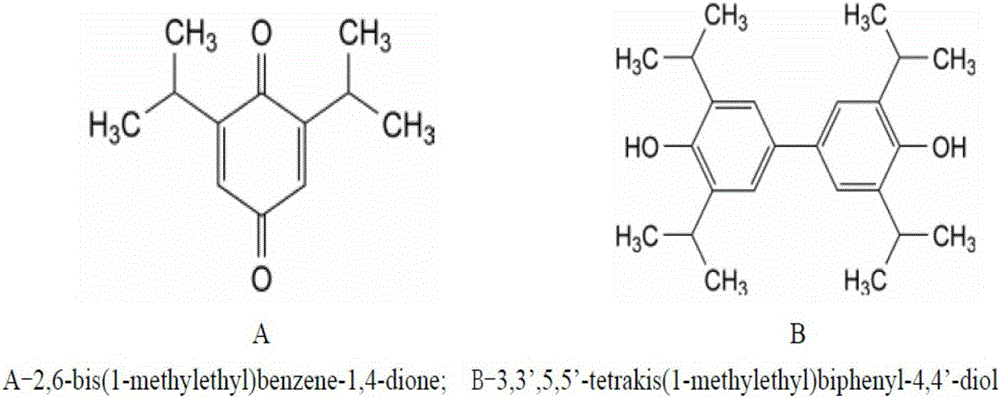
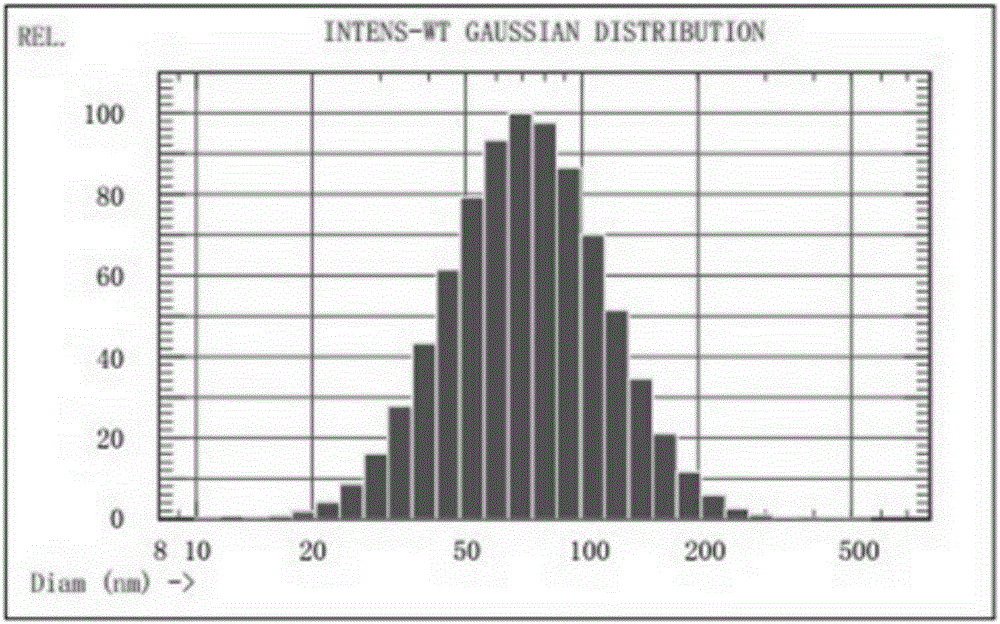
Propofol liposome freeze-drying preparation and preparation method thereof
A technology of freeze-dried preparations and propofol lipids, which is applied in the field of medicine, can solve the problems of easy merger of emulsion particles, bacterial and fungal infection, and easy infection, so as to achieve good encapsulation efficiency and stability, and improve quality and stability, reducing the effect of side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0059] The present invention screens and verifies the dosage range and preparation process of various auxiliary materials in the preparation formula of the non-irritating and painless propofol liposome freeze-dried preparation. The present invention is described in detail below in conjunction with embodiment, but not limited to following example.
[0060] 1. Preparation examples of non-irritating and painless liposome lyophilized formulations of propofol
[0061] (1) Non-irritating and painless propofol liposome freeze-dried preparations prepared by artificially synthesized phospholipids in different proportions
[0062] Example 1.1 Prescription:
[0063]
[0064] Preparation:
[0065] Step 1: Dissolve 1g of propofol, 21g of dipalmitoylphosphatidylcholine, 7g of dipalmitoylphosphatidylglycerol and 5g of cholesterol in absolute ethanol, stir to dissolve, and then depressurize the resulting solution in a water bath at 60°C Remove the organic solvent to prepare a uniform li...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com