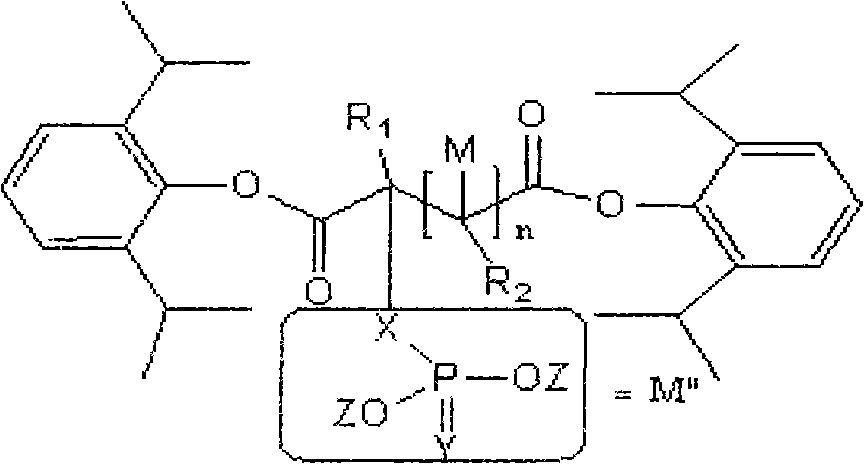
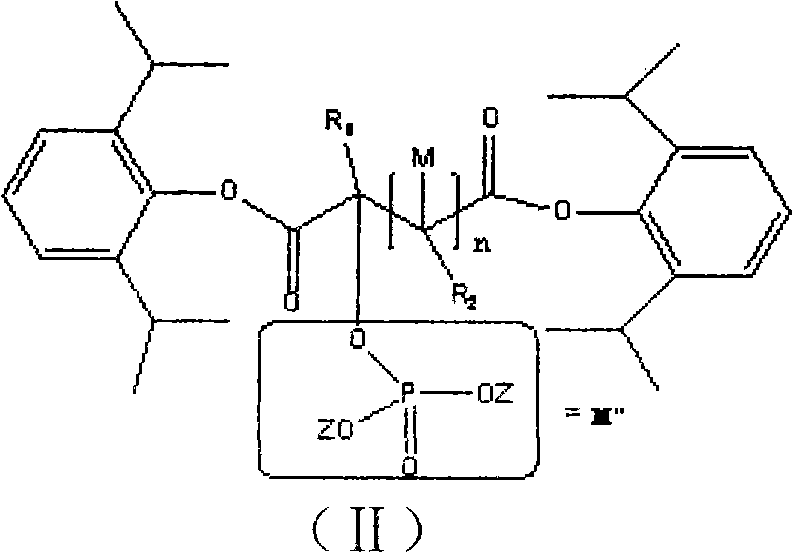
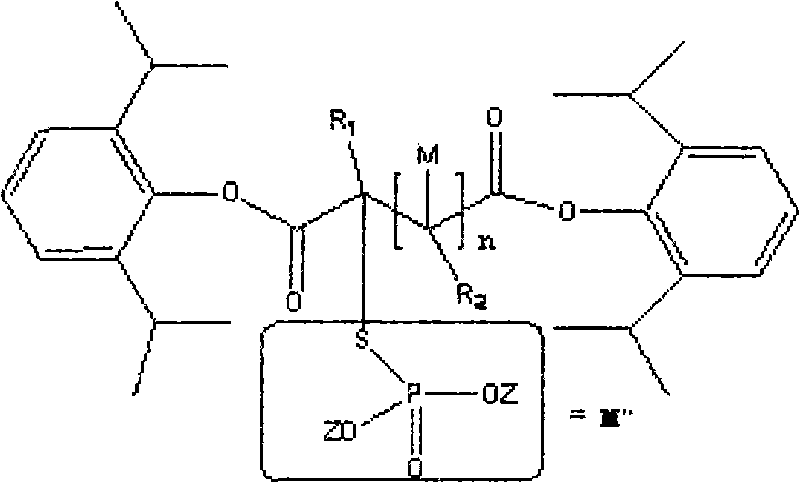
Water-soluble dipropofol and preparation method thereof
A dipofofol and water-soluble technology, applied in the field of water-soluble dipofol and its preparation, can solve the problems of increasing production cost, reducing product yield, small steric hindrance and easy generation of other by-products, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Synthesis of propofol 2-chloromalonate
[0027]
[0028] method one:
[0029] N 2 Under protection, add 16.4g (0.1mol) 2,6-diisopropylphenol, 200ml ether, 4. / g (0.2mol) sodium hydride in four-necked flask (with condenser tube and thermometer), react in 10 Stir at ℃ until there are no bubbles, then add 3.0 mol 2-chloromalonyl chloride dropwise to the reaction solution, track until the reaction is complete, filter the suspension, wash with ether, concentrate the ether under reduced pressure, and vacuum distill the remaining oil to obtain 2 - Propofol chloromalonate. The obtained substance is detected by a mass spectrometer (mass spectrometry data can provide information about the molecular weight and structure of the substance, and can qualitatively and quantitatively determine the sample). The obtained mass spectrometry data are, 1 H-NMR (D 6 -DMSO): δ1.29 (d, 24H, 8CH 3 ); 3.12 (m, 4H, 4CH); 5.10 (s, H, CH); 6.92 (m, 2H, 2CH); 7.21 (d, 4H, 4CH). The mass spect...
Embodiment 2
[0033] Synthesis of Dipropofol 2-Phosphate Sodium Malonate
[0034]
[0035] Add 0.1mol sodium phosphate, 0.1mol 2-propofol 2-chloromalonate and 100ml distilled water in the bottle, and magnetically stir under ice bath cooling. When the temperature in the bottle naturally drops to 15°C, add dropwise dimethyl sulfoxide ( DMSO) 80ml, the reaction temperature rises, control not to exceed 20°C, after adding, stir the reaction until complete, add 110ml of 95% ethanol dropwise to the solution, let it stand overnight, wash the obtained solid with alcohol once, and dry it in vacuum for 48h (40°C ), to get the product. The obtained substance is detected by a mass spectrometer (mass spectrometry data can provide information about the molecular weight and structure of the substance, and can qualitatively and quantitatively determine the sample). The obtained mass spectrometry data are, 1 H-NMR (D 6 -DMSO): δ1.29 (d, 24H, 8CH 3 ); 3.12 (m, 4H, 4CH); 5.14 (s, H, CH); 6.92 (m, 2H, 2C...
Embodiment 3
[0037] Synthesis of Propofol 2-chlorosuccinate
[0038]
[0039] method one:
[0040] N 2 Under protection, add 16.4g (0.1mol) 2,6-diisopropylphenol, 200ml ether, 4.8g (0.2mol) sodium hydride to a four-necked flask (equipped with a condenser tube and a thermometer), and react at 10°C Stir until there are no bubbles, then add 3.0mol 2-chlorosuccinyl chloride dropwise to the reaction liquid, track until the reaction is complete, filter the suspension, wash with ether, concentrate the ether under reduced pressure, and vacuum distill the remaining oil to obtain 2-chlorosuccinyl chloride Propofol Malonate. The obtained substance is detected by a mass spectrometer (mass spectrometry data can provide information about the molecular weight and structure of the substance, and can qualitatively and quantitatively determine the sample). The obtained mass spectrometry data are, 1 H-NMR (D 6 -DMSO): δ1.29 (d, 24H, 8CH 3 ); 2.75(d,2H,CH 2); 3.12 (m, 4H, 4CH); 4.94 (t, H, CH); 6.92...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com