Novel painless diluent, dilution compatibility method and application of alprostadil fat emulsion preparation
A technology of dil fat and alprostadil, which is applied in the field of diluents of new painless alprostadil fat emulsion preparations, can solve problems such as the harm of preparation stability and not a dilution method, and achieve the effect of reducing pain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
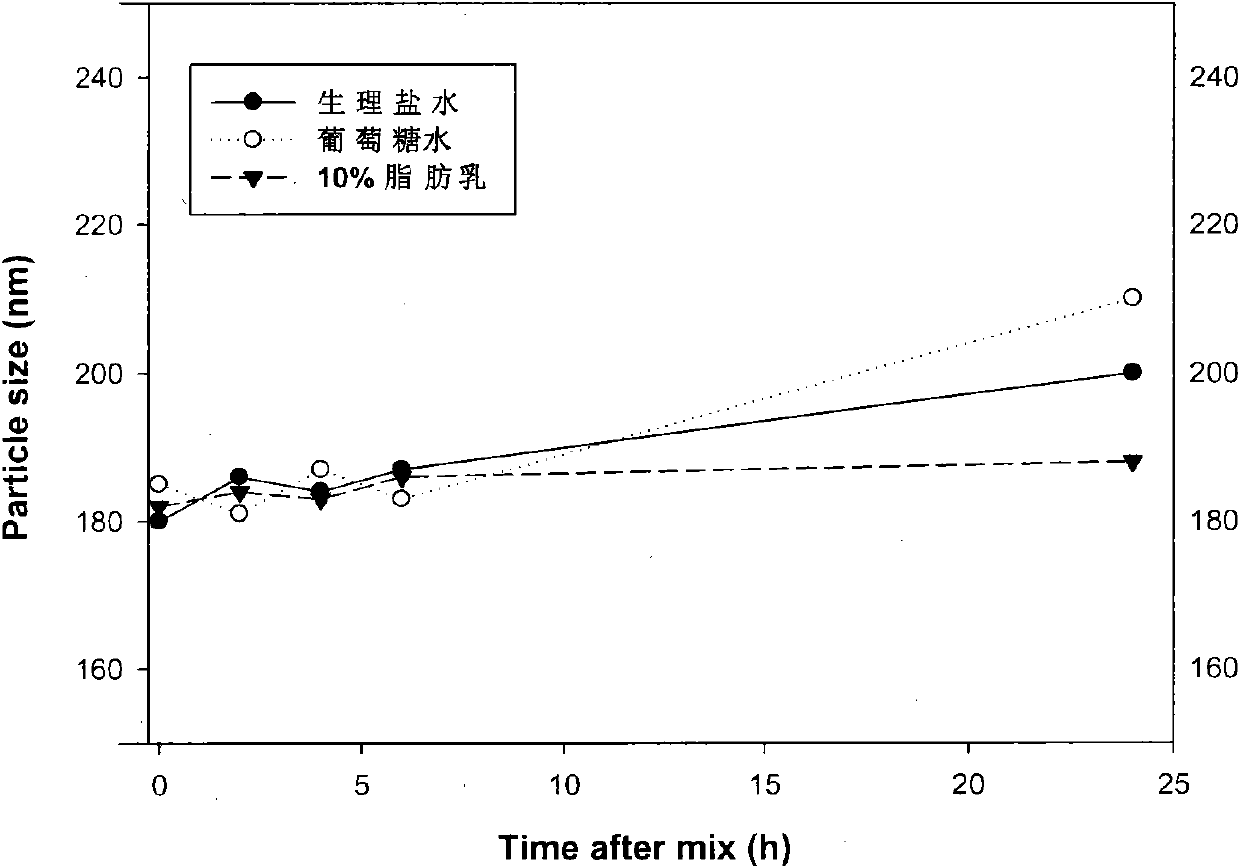
[0094] Embodiment 1: Physiological saline, injection glucose water and blank fat emulsion are investigated as diluents
[0095] Take 10ml each of physiological saline, glucose water for injection and 10% long-chain fat emulsion and mix with 2ml of alprostadil fat emulsion (5μg / ml) respectively. Place the container containing the mixed liquid in a water bath at 25°C, take samples according to the specified time, and measure the particle size of the mixed liquid. Such as figure 1 Shown, no matter diluting alprostadil fat emulsion with that diluent, the particle size of solution after mixing all still meets quality standard requirement. However, when glucose water and normal saline were used as diluents for a long time, the particle size of the mixture tended to increase, while when the blank fat emulsion was used as the diluent, the particle size of the mixture remained basically unchanged despite being left for 24 hours. It shows that it is more stable and reasonable to dilut...
Embodiment 2
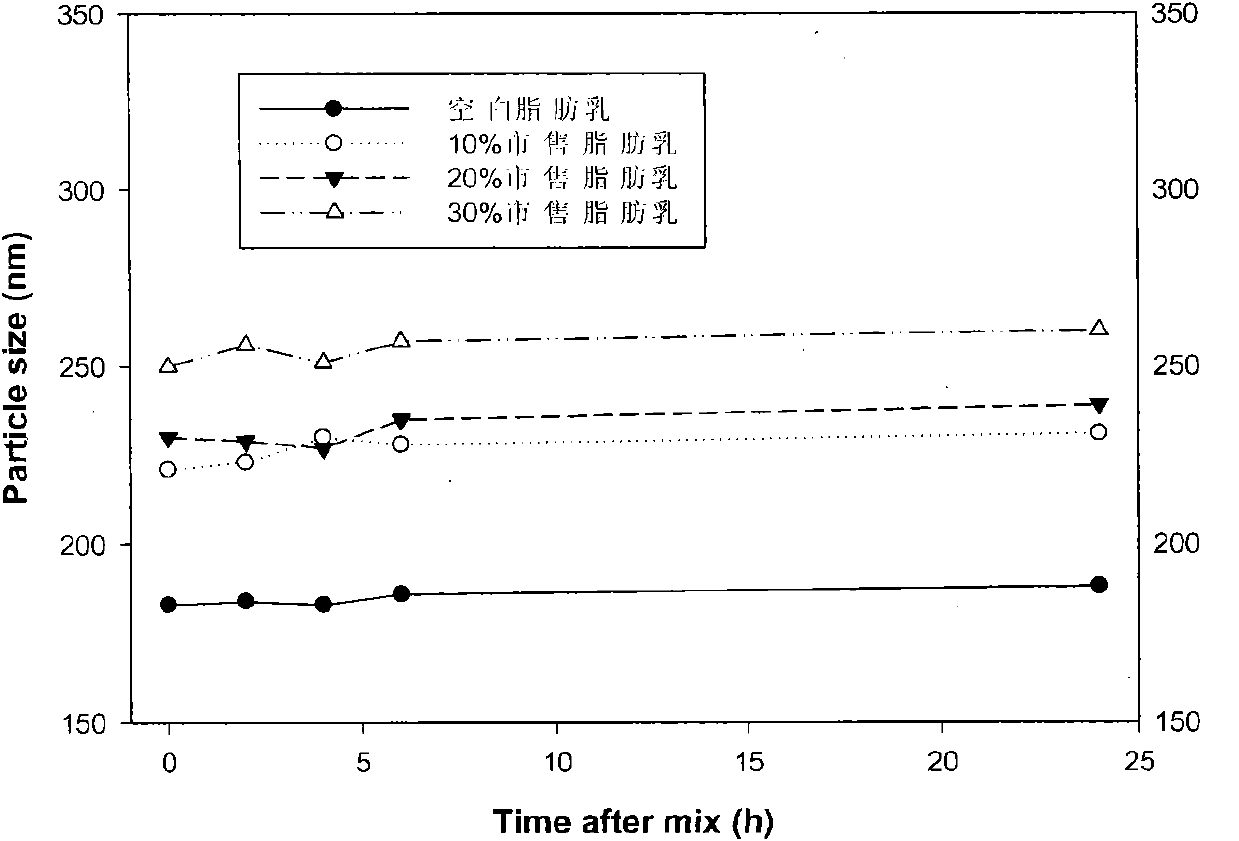
[0096] Embodiment 2: the investigation of blank fat emulsion, 10%, 20% and 30% commercially available fat emulsion as diluent
[0097] Take 10ml each of homemade blank fat emulsion and 10%, 20% and 30% commercially available long-chain fat emulsion and mix with 2ml of alprostadil fat emulsion (5 μg / ml) respectively. Place the container containing the mixed liquid in a water bath at 25°C, take samples according to the specified time, and measure the particle size of the mixed liquid. Such as figure 2 As shown, the particle size of the blank fat emulsion prepared by ourselves in this experiment is basically the same as that of the alprostadil lipid microspheres, so after mixing, the particle size of the mixed solution is consistent with that of the initial alprostadil lipid microspheres , while the particle size of commercially available fat emulsions is relatively large, and the diluent accounts for the majority of dilution, so the particle size after mixing is similar to tha...
Embodiment 3
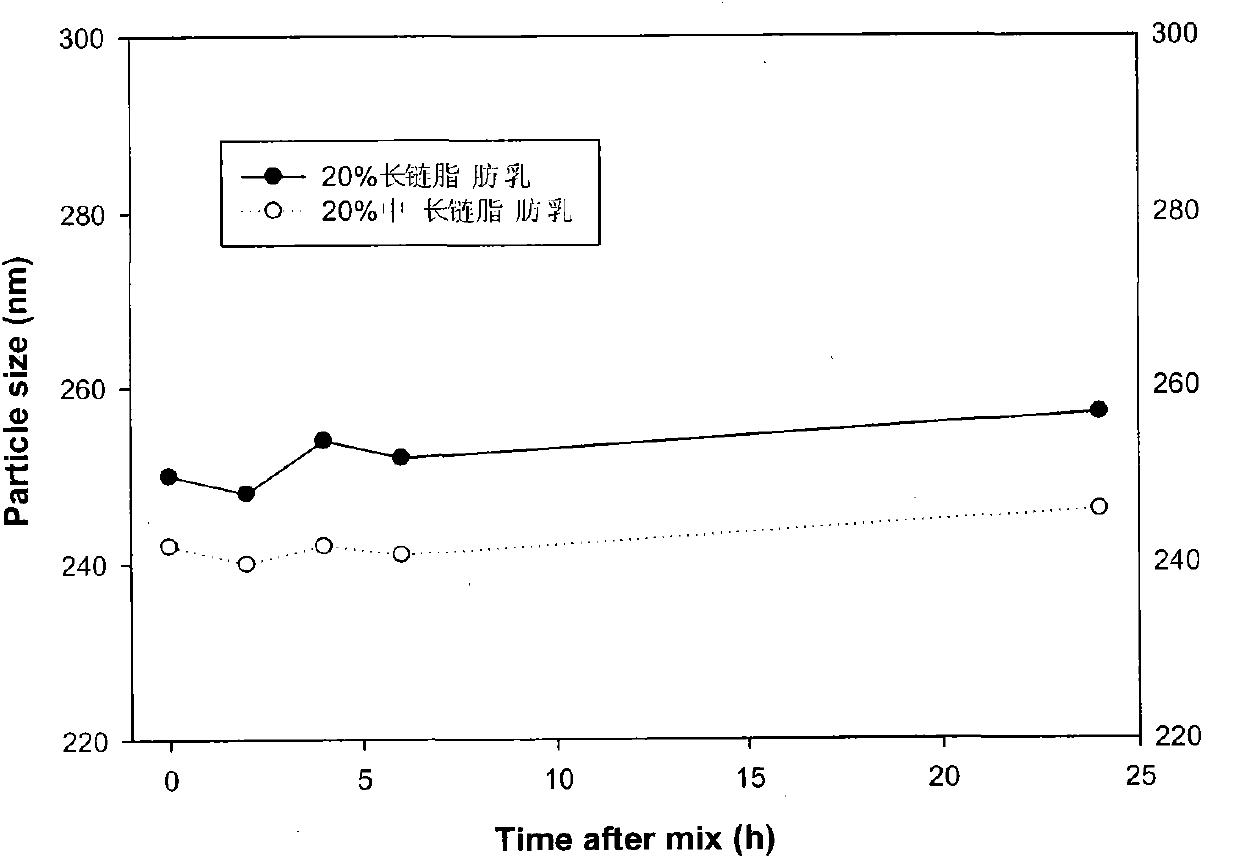
[0098] Example 3: Investigation of 20% long-chain and 20% medium-long-chain blank fat emulsion as diluent
[0099] 10 ml of commercially available 20% long-chain fat emulsion and 20% medium-long-chain fat emulsion were respectively mixed with 2 ml of alprostadil fat emulsion (5 μg / ml). Place the container containing the mixed liquid in a water bath at 25°C, take samples according to the specified time, and measure the particle size of the mixed liquid. Such as image 3 As shown, the changes and differences in the oil phase composition of fat emulsions have no essential difference for their use as diluents for alprostadil lipid microspheres. The particle size did not change significantly after dilution.
[0100] The second part, experimental investigation on the adsorption of free prostaglandin in water phase by blank fat emulsion
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com