Abiraterone inclusion compound tablet and preparation method thereof
A technology of abiraterone and inclusion compound, applied in the field of abiraterone inclusion compound tablet and preparation thereof, can solve the problems of poor compressibility of abiraterone, troubles in preparation development, poor water solubility of abiraterone, etc. The effect of poor solubility, accurate dosage and stable quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] The preparation of embodiment 1 abiraterone clathrate
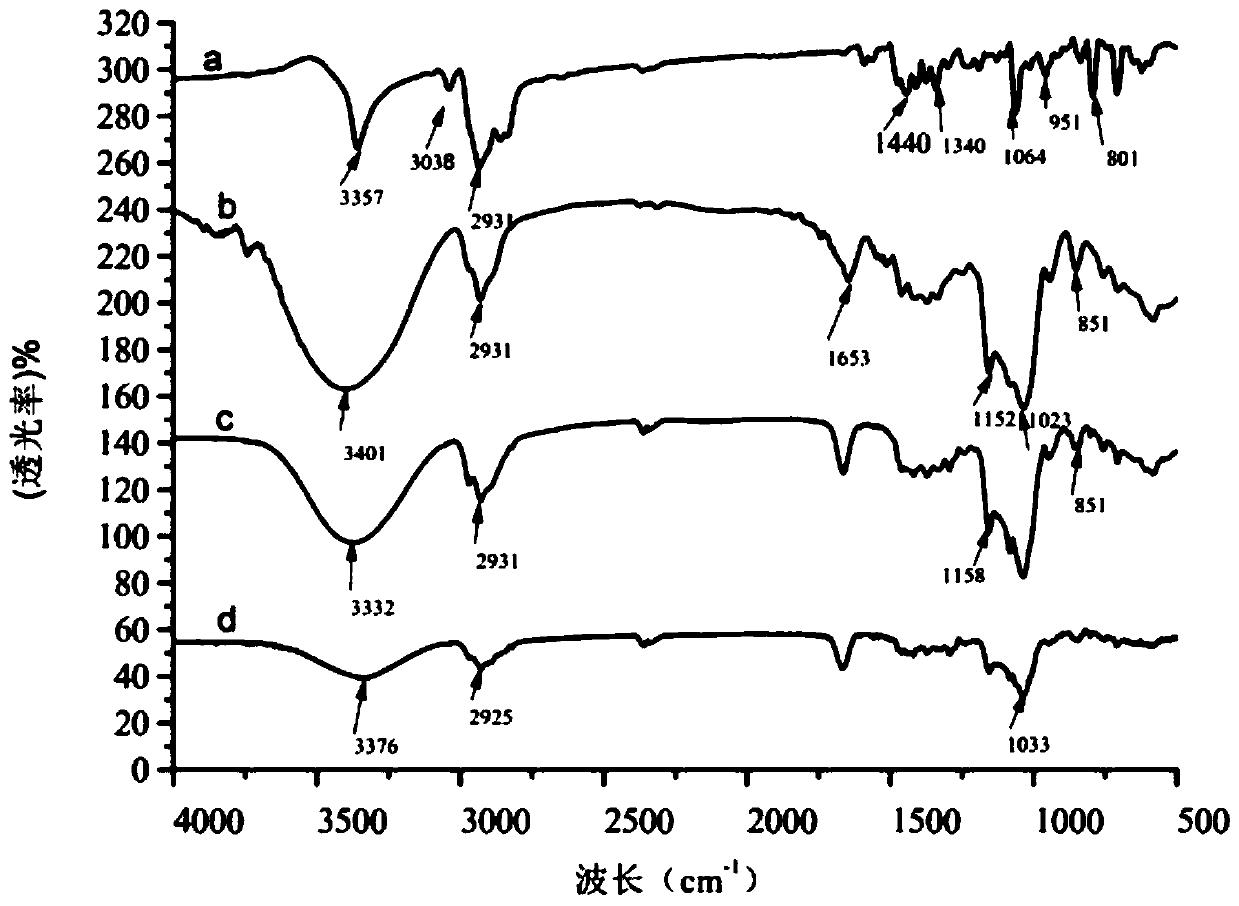
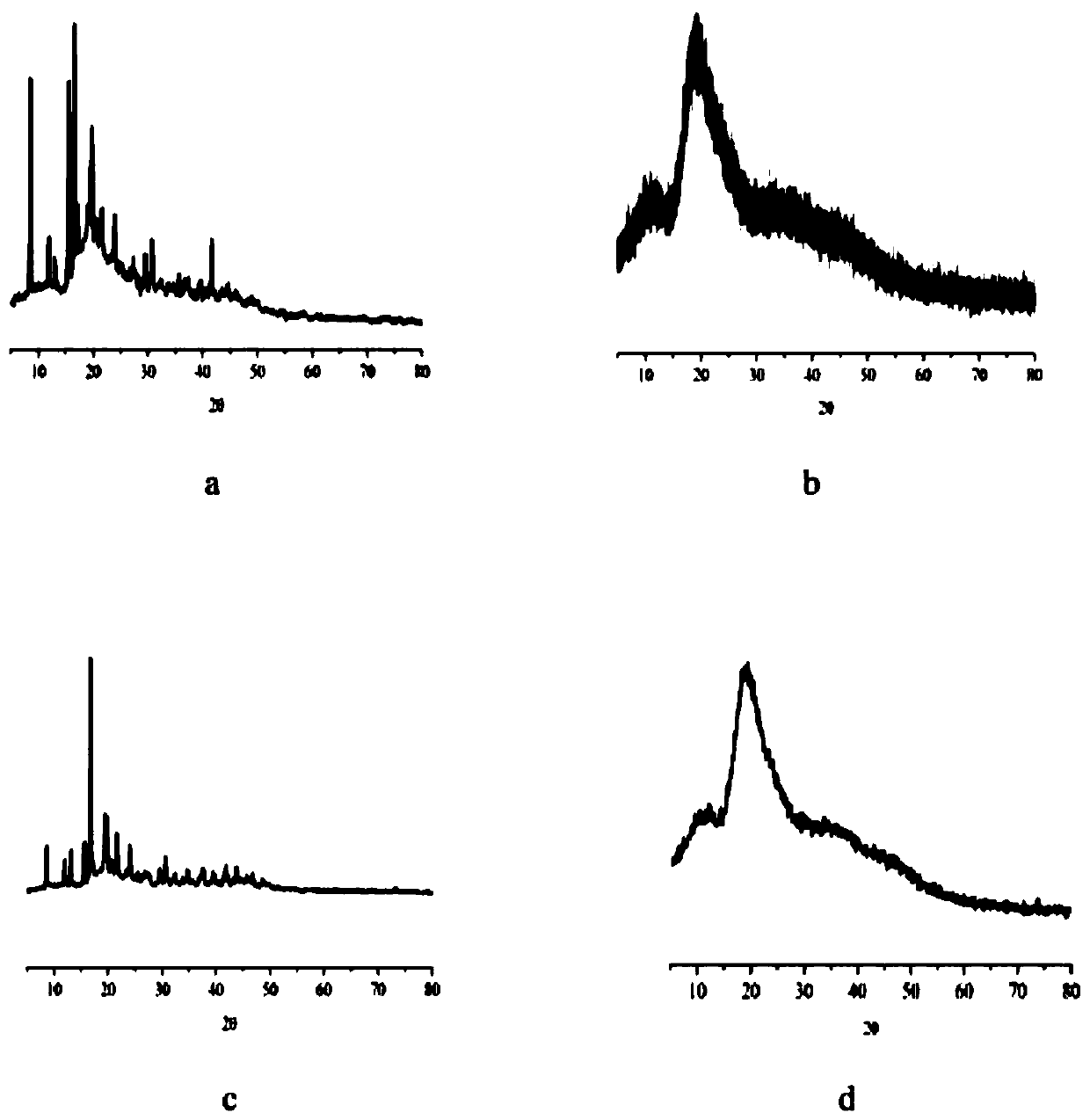
[0042] Precisely weigh 1 mol of abiraterone crude drug and put it into a 50ml reaction bottle, add 10ml of absolute ethanol, put it into an ultrasonic generator and ultrasonically oscillate for 30min until it is completely dissolved. Put it into a constant temperature magnetic stirrer with a set temperature of 75°C, weigh 3mol 2-HP-β-CD, and weigh 0.3mol polyvinylpyrrolidone into a 10ml stoppered test tube, add 5ml distilled water to dissolve it completely. Set the stirring speed of the constant temperature magnetic stirrer to 900r / min, slowly add the above-mentioned Abiraterone aqueous phase into the 2-HP-β-CD organic phase, stir for 6 hours, take it out, cool naturally, and remove the excess by rotary evaporation of the cooling liquid Absolute ethanol, put the suspension in the refrigerator at -10°C for 24 hours, fully analyze the clathrate, and then freeze-dry it in a freeze dryer at -50°C for 24 hours to obtain...
Embodiment 2
[0043] The characterization of embodiment 2 Abiraterone inclusion compound
[0044] 1.1 Determination of Encapsulation Efficiency
[0045] The inclusion compound yield was calculated by weighing, and the content of the inclusion compound was measured by ultraviolet light, so as to finally determine the encapsulation efficiency of the inclusion compound.
[0046] According to the preparation method of Example 1, four batches of Abiraterone inclusion compounds were prepared, and the encapsulation efficiency and drug content were shown in Table 1. The results showed that the Abiraterone inclusion compound encapsulated by the preparation method of Example 1 The ratio is between 52.72%-56.28%, and the drug content in the clathrate is between 19.26%-20.76wt%.
[0047] Table 1
[0048] batch number Encapsulation rate (%) Drug content (wt%) 1 56.28 19.91 2 54.94 20.18 3 52.72 20.76 4 54.78 19.26
[0049] 1.2 Solubility
[0050] For the det...
Embodiment 3~6
[0078] The preparation of embodiment 3~6 abiraterone inclusion compound tablet
[0079] Take Abiraterone inclusion complex and pharmaceutical excipients in proportion, and its specific ratio is shown in Table 5,
[0080] table 5
[0081] Abiraterone clathrate, % Thinner, % Disintegrant, % Binder, % Example 3 5 80 5 10 Example 4 25 59 15 1 Example 5 20 76 1 3 Example 6 20 70 5 5
[0082] Preparation:
[0083] (1) Weigh each component according to the above ratio, pass the abiraterone inclusion compound, diluent, and disintegrant through an 80-mesh sieve, and mix uniformly by the equal-volume incremental method;
[0084](2) Add binder to make soft material, pass the obtained soft material through a 14-mesh sieve to make wet granules, put it into a 60°C oven and dry until the moisture is 1-2%, pass through a 16-mesh sieve, and weigh the weight of the dry granules;
[0085] (3) Add 1% lubricant by weight of dry granules,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com