Medicinal composition containing cefixime cyclodextrin inclusion compound and preparation thereof
A technology of cyclodextrin inclusion compound and cefixime, which is applied in the direction of medical preparations containing active ingredients, medical preparations with non-active ingredients, nano-medicines, etc., to achieve high water solubility, easy dissolution, and small hemolytic side effects Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
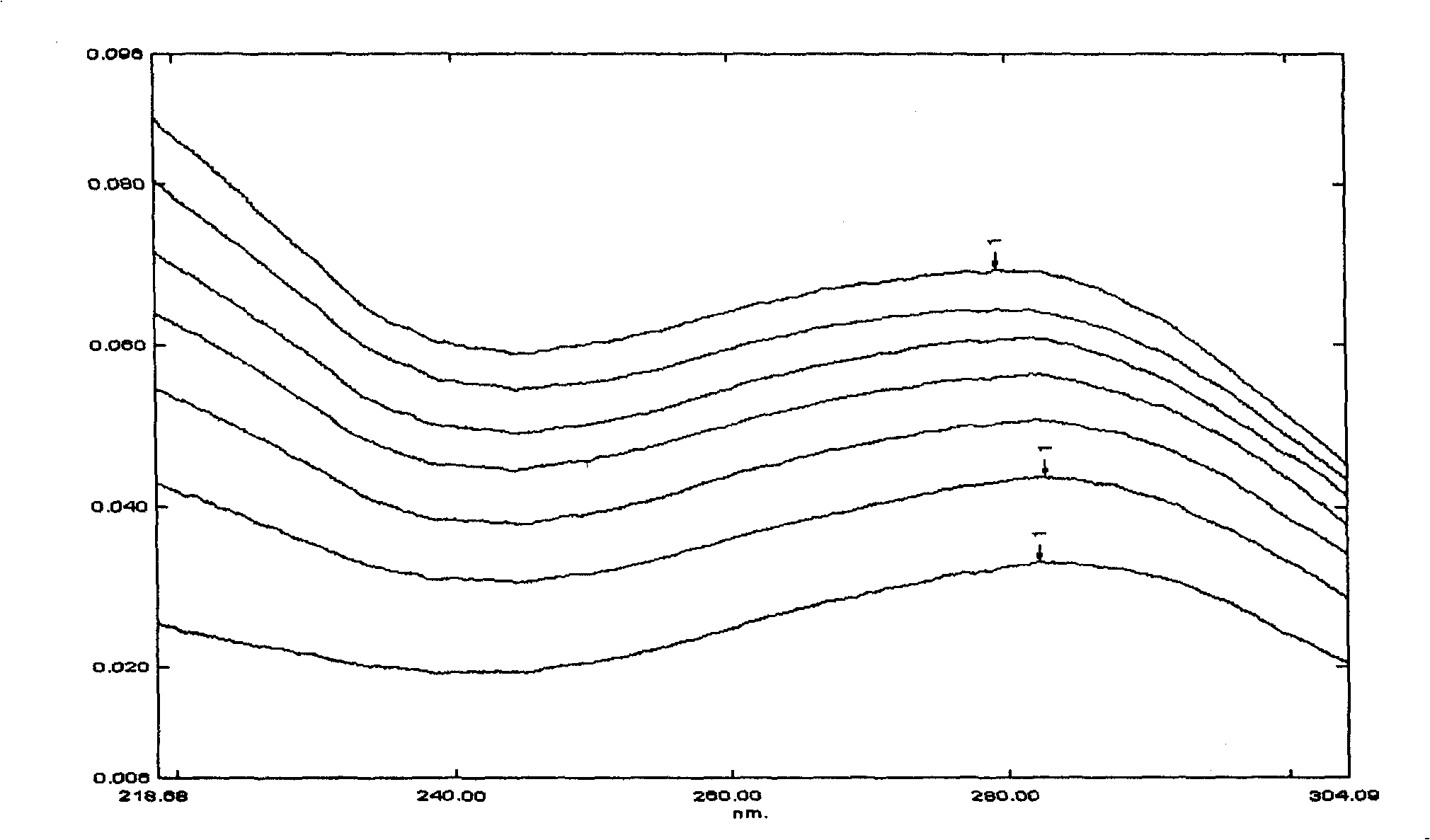
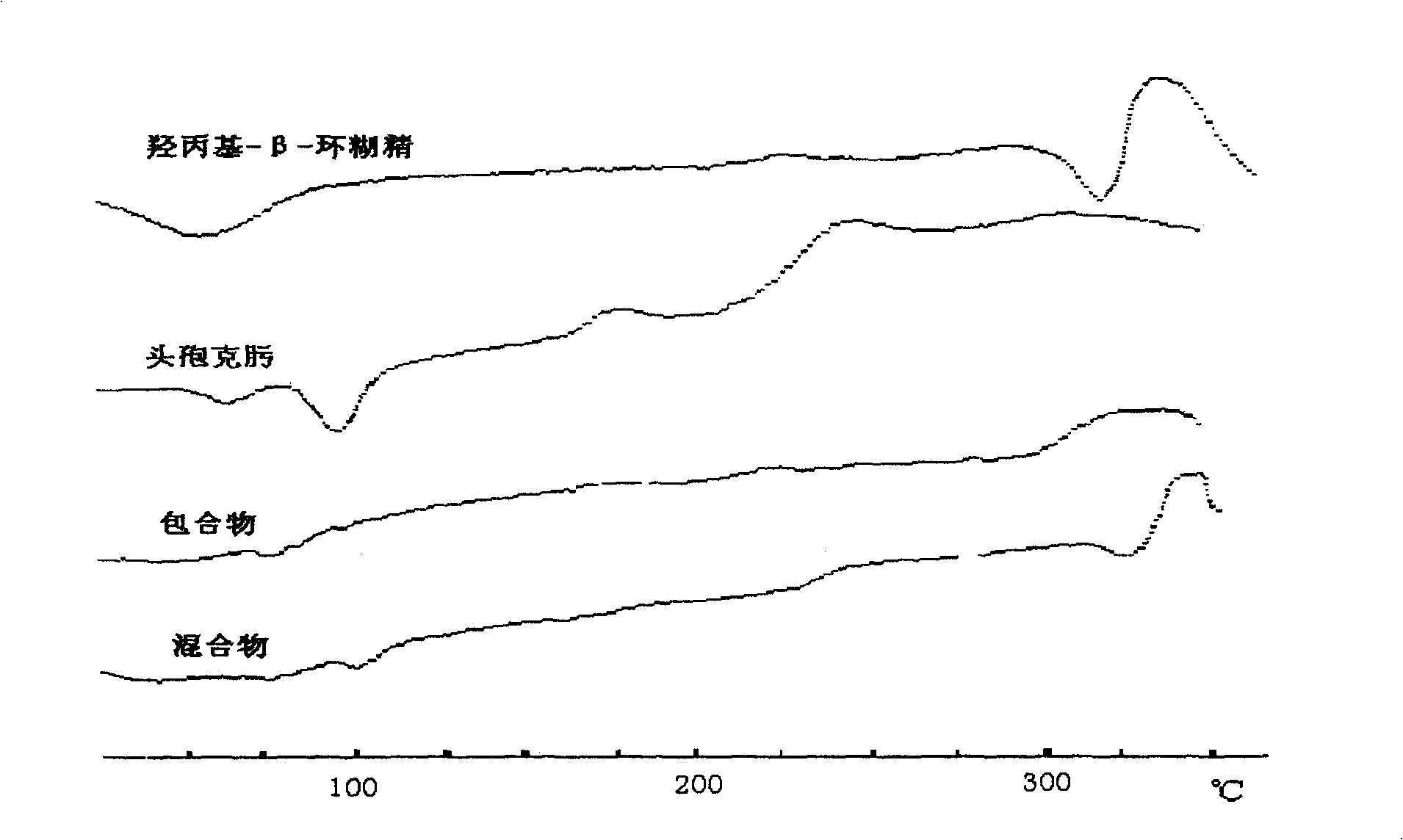
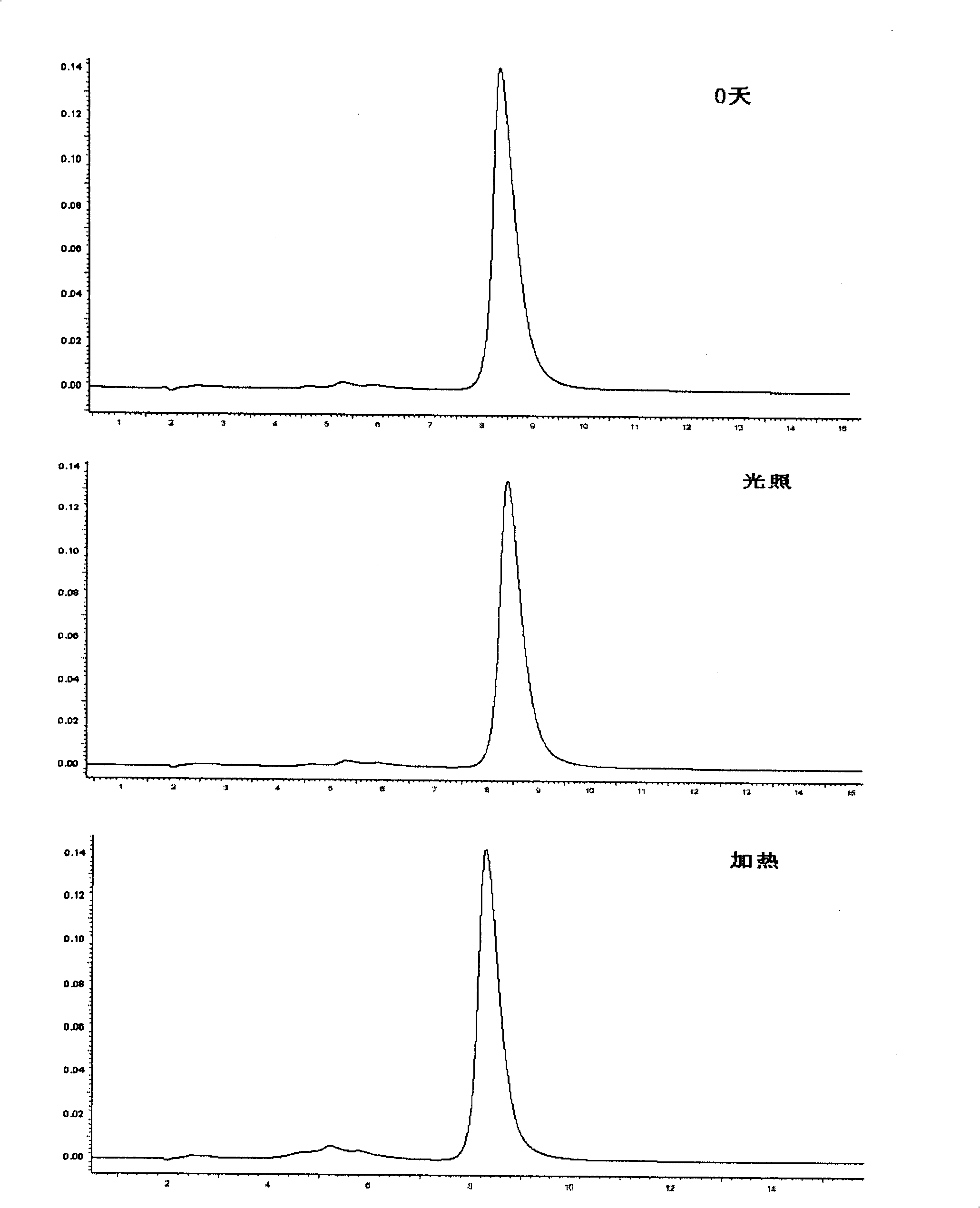
Image
Examples
Embodiment 1
[0071] Mix 10 grams of β-cyclodextrin with 10 milliliters of pure water, heat, add 4.0 grams of cefixime (equimolar) at a temperature of 50 ° C, mix and stir for 3 hours, then cool at 5 ° C for 24 hours; filter, Wash twice with water, and dry under pressure at room temperature to obtain a solid clathrate.
Embodiment 2
[0073] Mix 14 grams of hydroxypropyl-β-cyclodextrin with 20 milliliters of pure water, heat, add 4.0 grams of cefixime at a temperature of 50 ° C, add dilute alkali and mix thoroughly to dissolve, then add dilute acid to neutralize properties, stirring and cooling, and then 0.25 membrane filtration, 50 ° C drying under reduced pressure, that is, a solid clathrate.
Embodiment 3
[0075] Essentially the same as Example 2, but with 16 grams of sulfobutyl-β-cyclodextrin.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com