Dihydromyricetin cyclodextrin inclusion compound and preparation method thereof
A technology of dihydromyricetin and cyclodextrin is applied in the field of dihydromyricetin cyclodextrin inclusion complex and its preparation, and can solve the problem of low solubility and low bioavailability of β-cyclodextrin inclusion complex. problems, to achieve the effect of easy control of production conditions, improved solubility, and high content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] An inclusion compound of dihydromyricetin and cyclodextrin, including cyclodextrin and dihydromyricetin embedded in the cavity of cyclodextrin, forming dihydromyricetin as the object with cyclodextrin as the main molecule Molecular clathrates.
[0034] The cyclodextrin is one or two of α-cyclodextrin, β-cyclodextrin, γ-cyclodextrin and cyclodextrin derivatives and any combination thereof.
[0035] The cyclodextrin derivatives include: hydroxypropyl-β-cyclodextrin, glucose cyclodextrin, maltose cyclodextrin, dihydroxypropyl-β-cyclodextrin, maltotriose cyclodextrin, methyl- β-cyclodextrin, carboxymethylcyclodextrin, sulfanylcyclodextrin.
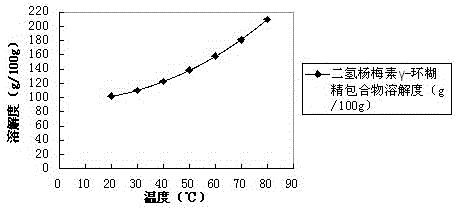
[0036] As a preferred solution of the previous step, the cyclodextrin is γ-cyclodextrin.
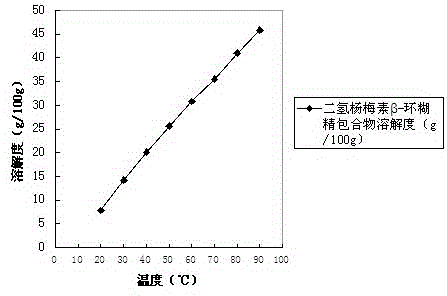
[0037] As a preferred solution of the previous step, the cyclodextrin derivative is hydroxypropyl-β-cyclodextrin.
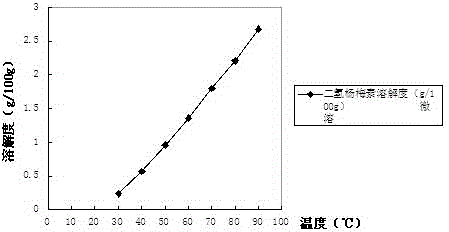
[0038] As a preferred solution in the previous step, the dihydromyricetin is dihydromyricetin extracted from plants of the genus...
Embodiment 2
[0041] A preparation method of cyclodextrin and dihydromyricetin inclusion complex, characterized in that it comprises the following steps:
[0042] (1) Dissolving 100-500 parts of dihydromyricetin in 100-39600 parts of solvent A to make a dihydromyricetin solution;
[0043] (2) Add 200-600 parts of cyclodextrin and / or cyclodextrin derivatives directly to the above dihydromyricetin solution and / or dissolve 200-600 parts of cyclodextrin and / or cyclodextrin derivatives In 100-39600 parts of solvent B, make cyclodextrin and / or cyclodextrin derivative solution and add the above dihydromyricetin solution;
[0044](3) Cut the mixed solution with a shearing machine, and when a complex is formed with a microscope, the inclusion is completed, and then spray-dried with a spray tower to obtain a powder solid dihydromyricetin and cyclodextrin and / or Cyclodextrin derivative inclusion compound
Embodiment 3
[0046] (1) Weigh 100-500 parts of dihydromyricetin and dissolve in 100-39600 parts of pure water, keep the dissolution temperature at 60-80°C to make dihydromyricetin suspension;
[0047] (2) Slowly add 200-600 parts of cyclodextrin or cyclodextrin derivatives to the above-mentioned dihydromyricetin suspension, and use a shearer at high speed to cut at 3000-8000r / min for inclusion, The temperature of the system is kept between 60-80°C, and the treatment time is 30-240min to make a dihydromyricetin inclusion compound solution;
[0048] (3) Spray-dry the above-mentioned dihydromyricetin inclusion compound solution with a spray tower at an inlet air temperature of 180-200°C and an outlet air temperature of 70-80°C to obtain dihydromyricetin cyclodextrin inclusion compound powder.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com