Method for preparing docetaxel/beta-cyclodextrin clathrates
A technology of cyclodextrin inclusion complex and docetaxel, which is applied in the directions of non-active components of polymer compounds, medical preparations containing active components, drug combinations, etc., to achieve the effects of improving inclusion rate and high solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] 1) Preparation of docetaxel / β-cyclodextrin inclusion compound
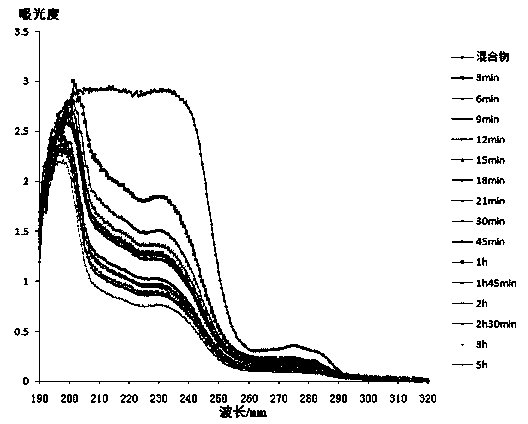
[0020] Weigh 100mg β-cyclodextrin with an analytical balance and place it in a 25ml volumetric flask, add pure water to constant volume to prepare a 4mg / ml β-cyclodextrin aqueous solution, weigh 25mg docetaxel and dissolve it in 1ml anhydrous methanol to prepare 25mg / ml Methanol solution of docetaxel. Take 5ml β-cyclodextrin aqueous solution and 0.04ml methanol solution of docetaxel in the autoclave successively, add a magnet, put the autoclave in a water bath at 35°C, turn on the magnetic stirring, and introduce carbon dioxide gas, when When the pressure in the autoclave reaches a certain pressure, the inlet valve is closed, and the autoclave is stabilized at 35° C. and 15 MPa, and stirred for four hours.
[0021] After the inclusion process in the supercritical high-pressure reactor is over, lower the temperature of the constant temperature water bath to room temperature, and gradually unscrew the air va...
Embodiment 2
[0033] Substantially the same as example 1, but wherein the supercritical reaction pressure is changed to 21MPa.
Embodiment 3
[0035]Substantially the same as example 1, but wherein the supercritical reaction pressure is changed to 25MPa.
[0036] in conclusion:
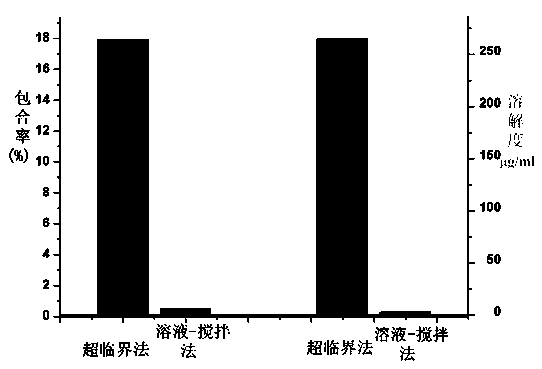
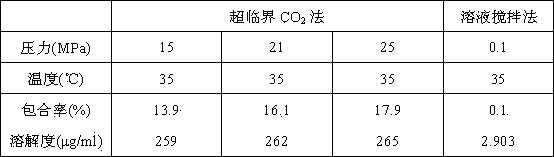
[0037] 1. The present invention proposes a method for preparing docetaxel / β-cyclodextrin inclusion compound using supercritical carbon dioxide fluid. By regulating the temperature and pressure of supercritical carbon dioxide fluid, the docetaxel / β-cyclodextrin Inclusion rate of docetaxel in fine clathrate. The results are shown in Table 1. The inclusion rate of docetaxel in the β-cyclodextrin inclusion compound is as high as 17.9%, much higher than the 0.1% encapsulation rate of the β-cyclodextrin inclusion compound prepared by the traditional method.
[0038] 2. The solubility of docetaxel / β-cyclodextrin inclusion compound prepared by supercritical carbon dioxide method in water is higher than that of docetaxel / β-cyclodextrin inclusion compound obtained by conventional solution-stirring method , the results are shown in Table 1. The solub...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com