Method for solubilizing camptothecin compound
A technology for camptothecin and compounds, which is applied in the field of solubilization of camptothecin compounds, can solve problems such as poor water solubility, and achieve the effect of high solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Add 10ml of 0.4M tartaric acid solution to 1gHP-β-CD, and vortex to dissolve to obtain a 10% HP-β-CD solution; add 5ml of 0.4M NaOH solution to 20mg of 10-hydroxycamptothecin, and vortex to dissolve, the alkaline solution of the drug Add 5ml of 10% HP-β-CD solution to the solution, vortex and shake until it is clear and transparent to obtain a clathrate solution with a concentration of about 2mg / ml, and the 10-hydroxycamptothecin in the solution can be detected by HPLC The purity is 98.6%.
Embodiment 2
[0046] Add 10ml of 0.55M hydrochloric acid solution to 2g of SB-β-CD, vortex to dissolve to obtain 20% HP-β-CD solution; add 5ml of 0.5M NaOH solution to 10mg of SN38, vortex to dissolve, add 5ml of 20% SB to the alkaline solution of the drug - β-CD solution, vortex and shake until clear and transparent, if necessary, adjust the pH value to 3.5 with the corresponding acid or alkali solution to obtain an inclusion complex solution with a concentration of about 1mg / ml, and divide the solution into penicillin bottle, freeze-dried to obtain SN38 freeze-dried powder. Through HPLC detection, it can be measured that the purity of SN38 in the freeze-dried powder is 99.2%.
Embodiment 3
[0048]
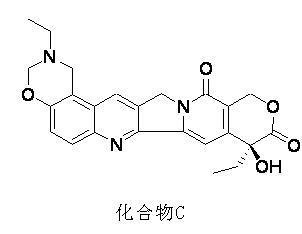
[0049] Add 3g of SB-β-CD to 10ml of 0.25M citric acid solution, vortex to dissolve to obtain a 30% SB-β-CD solution; add 5ml of 0.2M NaOH solution to 40mg of compound A, vortex to dissolve, add 5ml to the alkaline solution of the drug 30% SB-β-CD solution, vortex and shake until clear and transparent, and leave it for 2 hours to obtain a clathrate solution with a concentration of about 4 mg / ml. Through HPLC detection, the purity of compound A in the solution can be measured to be 97.8%. .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com