L-isovalerylspiramycin iii, its preparation, preparation method and application
A technology of L-isovaleryl spiramycin and rot-isovaleryl spiramycin is applied in the field of macrolide genetic engineering new antibiotics, and can solve problems such as difficulty in reaching chemical quality control standards and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] Example 1 Separation and preparation of L-isovalerylspiramycin III
[0085] (1) Biological fermentation: the helicase-producing bacteria clone strain WSJ-195 containing the 4″-isovaleryltransferase gene was mixed with soybean meal 2%, glucose 1%, starch 3%, CaCO 3 0.5%, NaCl 0.4% and agar 2% on the slant medium, cultured at pH 6.5-7.5, temperature 28-38 ℃ for 8-15 days, inoculated with soybean cake powder 1.5%, starch 3.0%, NaCl 0.4%, CaCO 3 0.5%, fish peptone 0.3% and KH 2 PO 4 0.05% seed medium, cultured at pH 6.5-7.5, 25-30°C for 40-80 hours, inoculated with 0.1-20% inoculum containing 0.5% glucose, 6.0% starch, 0.5% yeast powder, Fishmeal 2.0%, NH 4 NO 3 0.6%, NaCl 1.0%, CaCO 3 0.5%, KH 2 PO 4 0.05%, MgSO 4 0.1%, 0.5% soybean oil and 0.02% anti-foaming agent fermentation medium, cultivated under the conditions of pH 6.5-7.5, 26-30 ℃ for 72-120 hours to obtain the fermentation liquid;
[0086] Among them, through the adjustment and optimization of the...
Embodiment 2
[0098] Embodiment 2: Separation and preparation of L-isovalerylspiramycin III
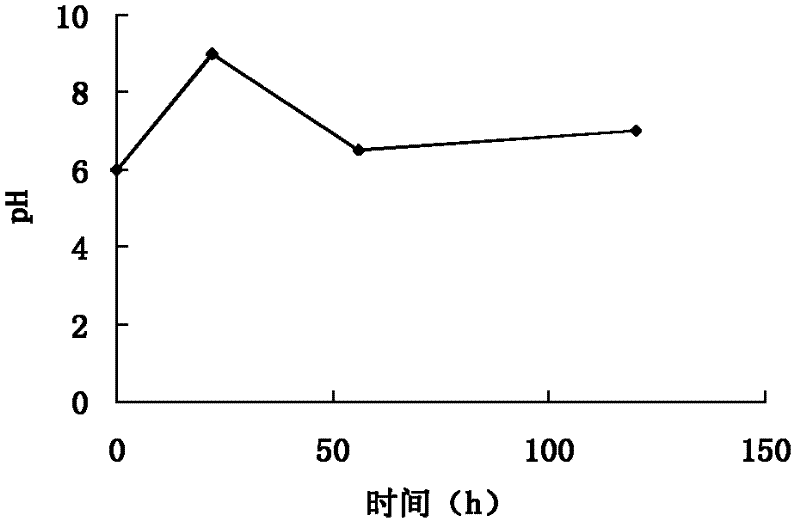
[0099] (1) Biological fermentation: the helicase-producing bacteria clone strain WSJ-195 containing the 4″-isovaleryltransferase gene was mixed with soybean meal 2%, glucose 1%, starch 3%, CaCO 3 0.5%, NaCl 0.4% and agar 2% on the slant medium, cultured at pH 7.2, temperature 32 ℃ for 12 days, inoculated with soybean meal 1.5%, starch 3.0%, NaCl 0.4%, CaCO3 0.5 %, 0.3% fish peptone and 0.05% KH2PO4 seed medium, cultured at pH 7.2 and 27°C for 70 hours, planted with 12% inoculum containing 0.5% glucose, 6.0% starch, 0.5% yeast powder, Fishmeal 2.0%, NH4NO3 0.6%, NaCl 1.0%, CaCO3 0.5%, KH2PO4 0.05%, MgSO4 0.1%, soybean oil 0.5% and defoamer 0.02% of the fermentation medium, at pH6.0~9.0, 26℃ Cultivate under high temperature for 100 hours to obtain fermentation broth; ferment under the condition of pH 6.0-8.0, the fermentation time is 110h, and the change curve of pH value with time presents three c...
Embodiment 3
[0111] Example 3 Separation and preparation of L-isovalerylspiramycin III
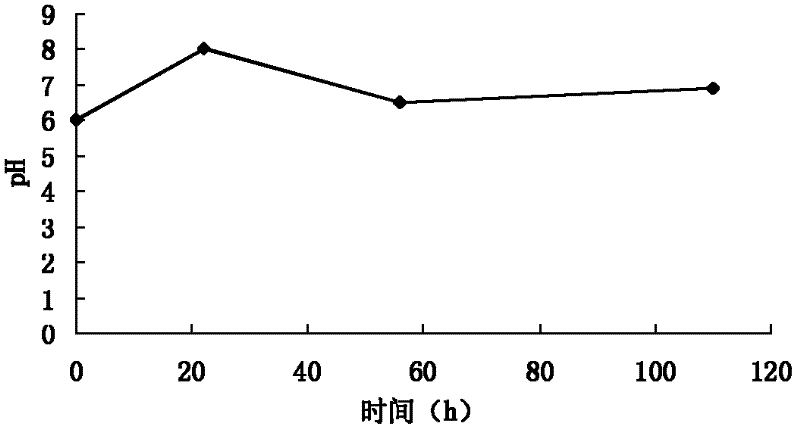
[0112] (1) Cultivation and fermentation: the spiramycin-producing bacteria clone strain WSJ-195 containing the 4″-isovaleryltransferase gene is cultivated on the slant medium, and then inoculated into the seed medium. After cultivation, the It is inoculated in the fermentation medium, the fermentation process is controlled by glucose and citric acid, and the fermentation is carried out under the condition of pH 6.0-7.5. The fermentation time is 115 hours, and the change curve of pH value with time presents three consecutive stages. One stage satisfies the equation y 1 =0.0682x 1 +6.0, where 01 2 =-0.0294x 2 +8.147, where 222 3 =0.0078x 3 +6.06, where 563 Figure 4 , to obtain the fermentation broth.
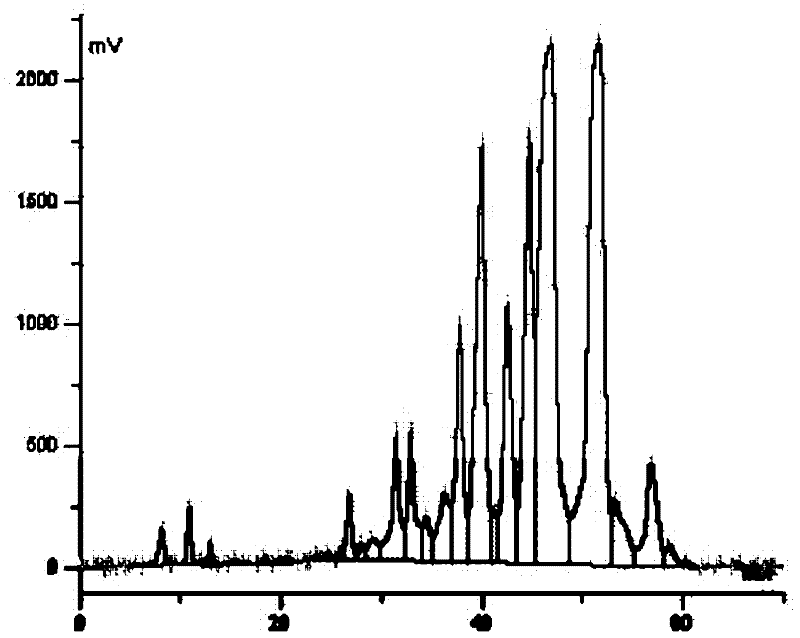
[0113] (2) Extraction of biological fermentation liquid: the fermentation liquid that step (1) obtains is treated with aluminum sulfate to obtain filtrate, adjusts pH to 8.6, extracts with butyl aceta...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com