Separation and preparation of isovaleryl-spiramycin I and application thereof
A technology of isovalerylspiramycin and compounds, which is applied in the separation and preparation of isovalerylspiramycin I and its application field, can solve the problem of rare multi-component antibiotic injections, increase the difficulty of quality control standards for final products, and Fundamental changes and other issues, to achieve rapid results, simplified production process, easy to accept the effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
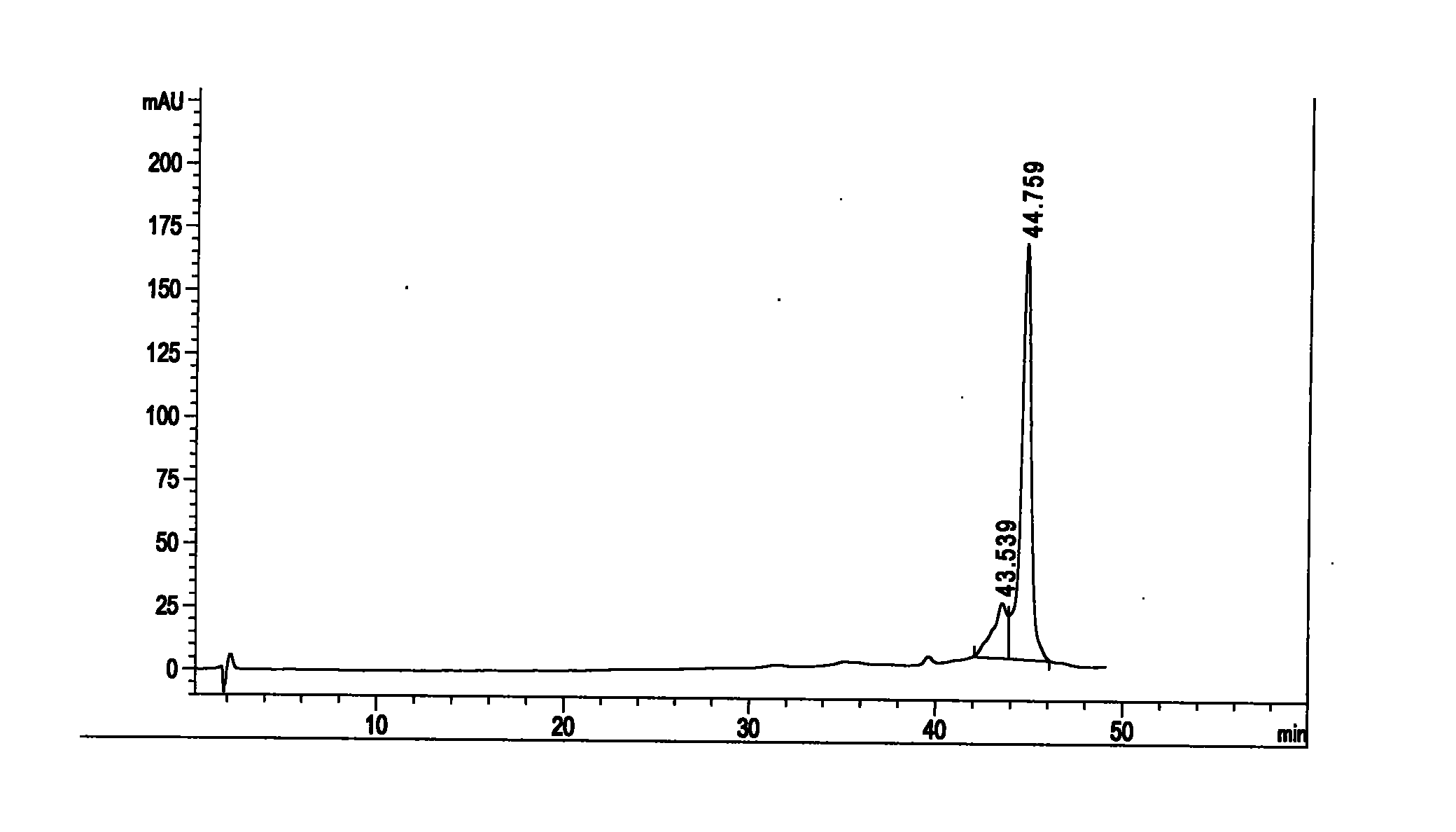
[0049] , the separation and preparation of isovalerylspiramycin I
[0050] The raw material of carrimycin is prepared according to the patent of "A Method for Manufacturing Shengjimycin Using Genetic Engineering Technology" (patent number: ZL97104440.6). The corimycin sample was separated by preparative high-performance liquid chromatography to obtain the pure product of isovalerylspiramycin I compound. Specifically, it includes rough separation of samples, high-efficiency purification, sample post-processing and other steps.
[0051] 1) Rough separation: first, the isovalerylspiramycin I component is roughly separated from the carrimycin sample, using silica gel column chromatography, using ethyl acetate and methanol as eluents, and three times respectively Column volume ethyl acetate: methanol (3:1) and three to five column volumes ethyl acetate: methanol (1:1) for elution, collect ethyl acetate: methanol (1:1) fraction, and concentrate the collected solution To dryness, t...
Embodiment 2
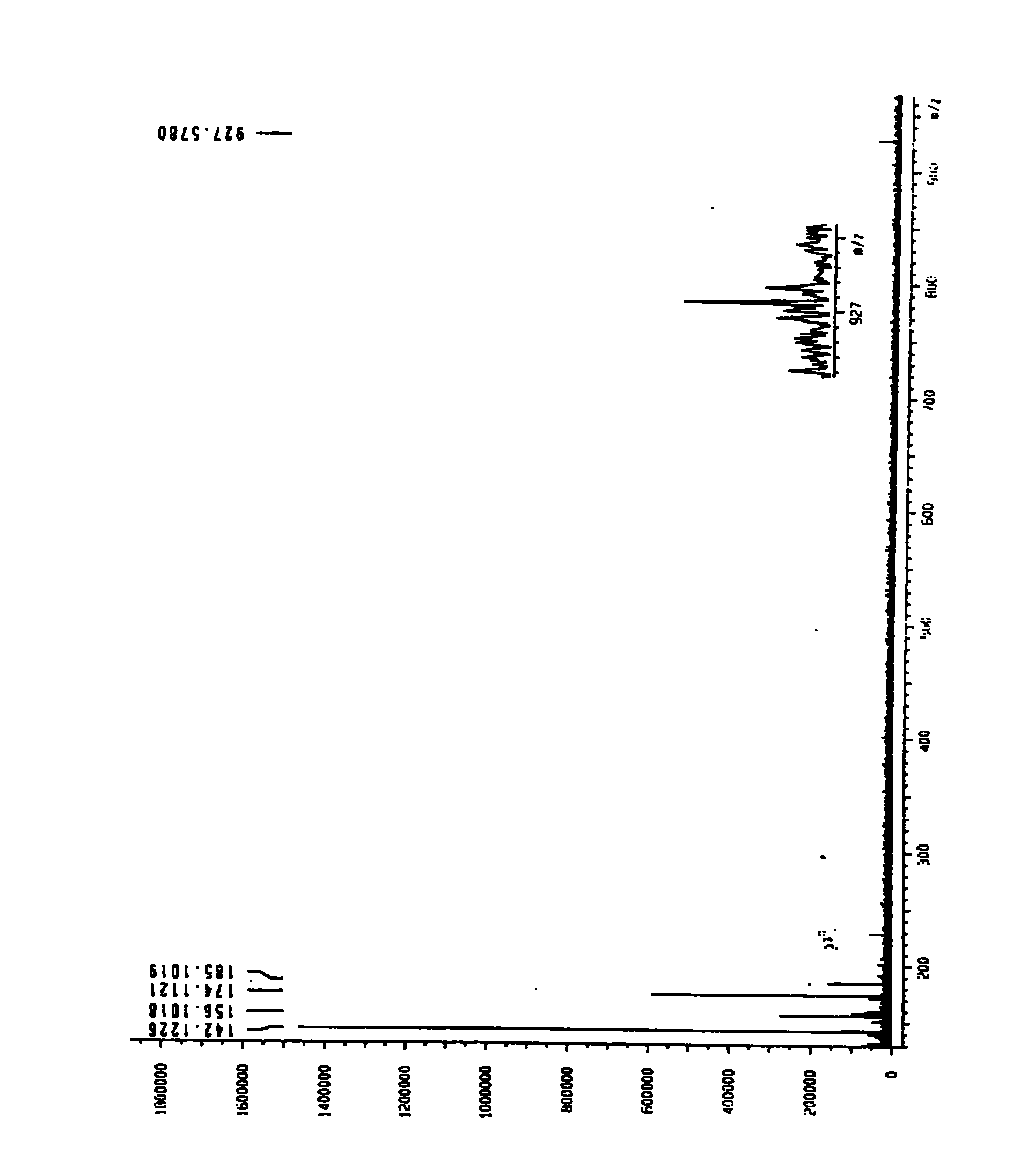
[0056] Identification of Isovalerylspiramycin I
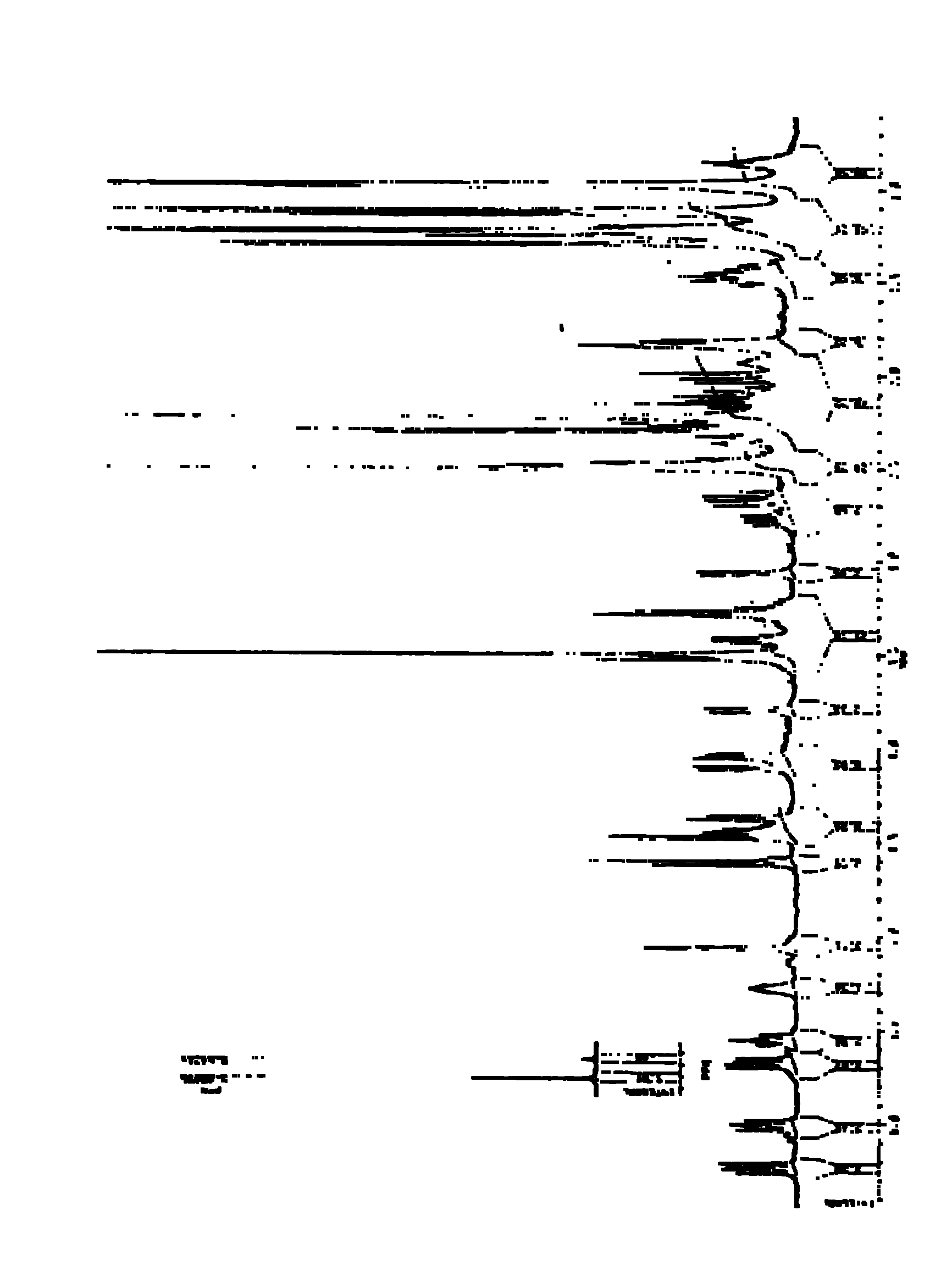
[0057] The molecular weight of the isolated compound was confirmed to be 926 by high-resolution mass spectrometry (Bruker APEXII, HR-SI-MS). Isovalerylspiramycin I high resolution mass spectrum see ( figure 2 ), by NMR 1 H and 13 C spectrum [BrukerAM500, solvent CDCl 3 , internal standard TMS (tetramethylsilane)] and other analyzes confirmed that the structure of the isolated compound is isovalerylspiramycin I. Isovalerylspiramycin I NMR 1 H and 13 C spectrum see ( image 3 , 4), NMR 1 H and 13 See C spectrum data (Table 1, 2).
[0058] Table 1 NMR of isovalerylspiramycin I 1 H spectrum data (500MHz, CDCl 3 )
[0059] Proton
[0060] Proton
[0061] Proton
[0062] The nuclear magnetic resonance of table 2 isovalerylspiramycin I 13 C spectrum data (125MHz, CDCl 3 )
[0063] Carbon
[0064] Carbon
Embodiment 3
[0065] the antibacterial activity assay of isovalerylspiramycin I
[0066] A antibacterial activity
[0067] 1) Compound samples:
[0068] Compound 1 carrimycin: batch number: 0812512, potency: 92.8%, provided by Shenyang Tonglian Group Co., Ltd.;
[0069] Compound 2 Isovalerylspiramycin I: batch number: 0811214, potency: 93.7%, provided by Shenyang Tonglian Group Co., Ltd.;
[0070] Erythromycin: batch number: 0706392, potency: 91.6%, China National Institute for the Control of Pharmaceutical and Biological Products;
[0071] Azithromycin: batch number: 0421-9603, potency: 99.3%, China National Institute for the Control of Pharmaceutical and Biological Products;
[0072] Clarithromycin: batch number: 0482-9901, potency: 90.4%, China Institute for the Control of Pharmaceutical and Biological Products.
[0073] 2) Test strains:
[0074] Standard strain: Streptococcus pneumoniae ATCC49619;
[0075] Gram-positive bacteria isolated clinically;
[0076] Erythromycin-resista...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com