Application of macrolide antibiotics in blocking influenza virus infection
An influenza virus infection, macrolide technology, applied in the direction of antiviral agents, medical preparations containing active ingredients, amine active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
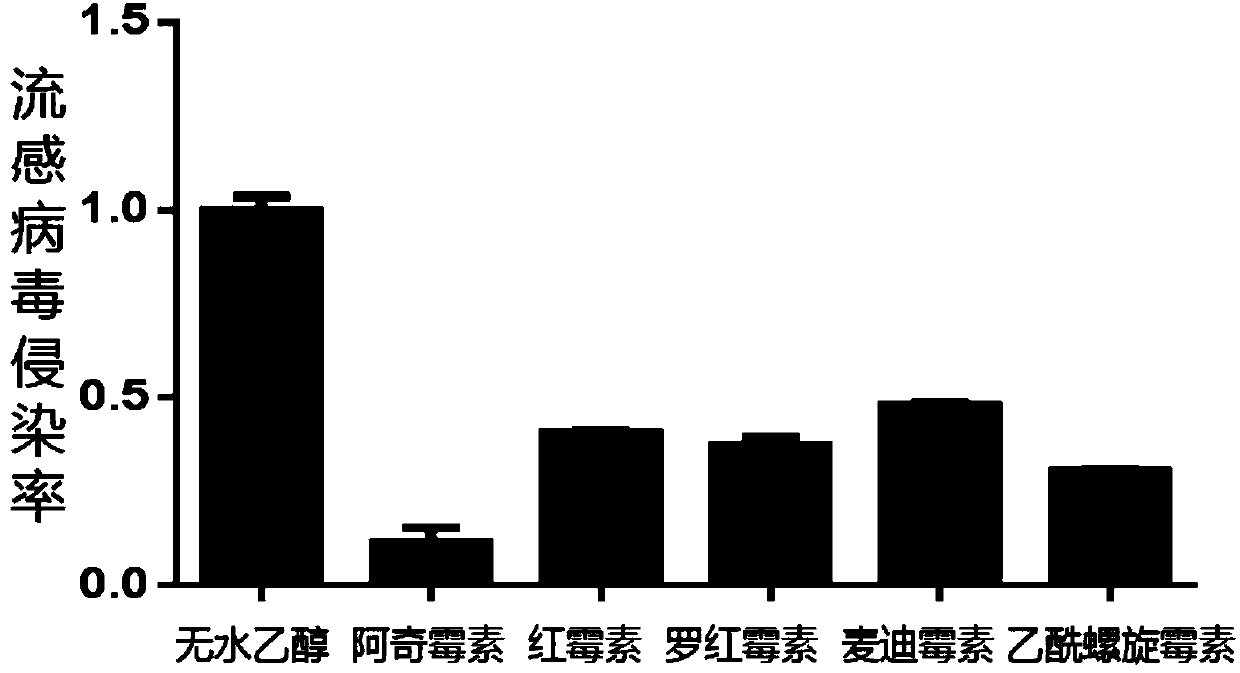
[0060] Example 1 : Macrolide antibiotics have the ability to resist the infection of influenza A virus A / PuertoRico / 8 / 34(PR8)-Luc
[0061] A549 cells (human lung cancer epithelial cells) are an ideal cell line for studying influenza virus. The inventors used the IAV-Luc influenza virus model with the luciferase reporter gene constructed by the research group of Chen Ling, Chinese Academy of Sciences using a reverse genetic system ( Using influenza virus PR8 as the backbone, a humanized luciferase gene is added to the C-terminus of the NA coding gene) against a number of marketed small molecule antibiotics (including the 14-membered ring macrolide antibiotic erythromycin, erythromycin 15-membered ring macrolide antibiotic azithromycin and 16-membered ring macrolide antibiotic acetylspiramycin, midecamycin) were evaluated for anti-influenza virus infection ability. The inventor first seeded A549 cells in a 96-well plate at 10,000 cells per well, incubated the drug 16 hours lat...
Embodiment 2
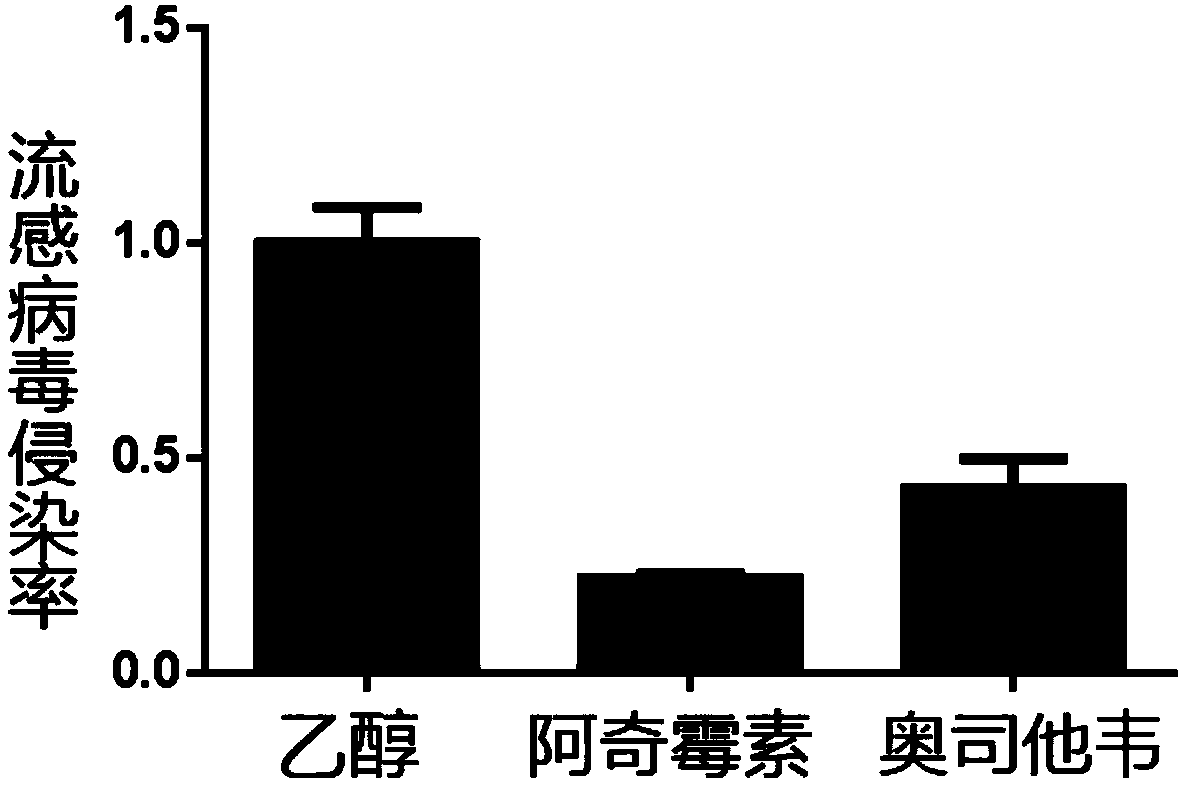
[0063] Example 2 : Comparison of Azithromycin and Oseltamivir Anti-Influenza Virus Infection
[0064] Since azithromycin often acts as an adjuvant when oseltamivir treats influenza virus in the prior art, the ability of azithromycin and oseltamivir to resist influenza virus infection was compared separately. Using the same experimental method steps as in Example 1, the anti-infection capabilities of azithromycin and oseltamivir were compared.
[0065] Such as image 3 As shown, after data analysis, it was found that azithromycin had stronger anti-influenza virus infection ability than oseltamivir at the same concentration (10 μM).
Embodiment 3
[0066] Example 3 : Azithromycin can prevent Barb / C mice against A / WSN33 / H1N1
[0067] Twenty-four female Barb / C mice aged 6-8 weeks were randomly divided into 4 groups (A, B, C, D) with 5 mice in each group. Ear piercings of all animals were marked, and then treated with designated preparations: group A was a blank control group; group B was a challenged PBS group, group C was a challenged ribavirin group, and group D was a challenged azithromycin group. Two days before the challenge, 50mg / kg of ribavirin and 50mg / kg of azithromycin were administered orally, and on the third day, 40 LD 50 (10 3 PFU / ml) dose of influenza virus intranasally infected mice, each 50 μl, the blank group was replaced by PBS, administered continuously for 5 days after the challenge, the administration method was the same as above, after fasting for 12 hours, weighed, The lungs were aseptically weighed after eyeball bleeding, and the lung index was calculated to evaluate the protective effect of azit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com