Oral composition of spiramycin and preparation method thereof
A technology of spiramycin and composition, which is applied to the oral composition of spiramycin and the field of preparation thereof, can solve the problems of poor taste-correcting effect, cumbersome process and the like, achieves no influence on drug bioavailability, low cost, good quality effect of taste
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
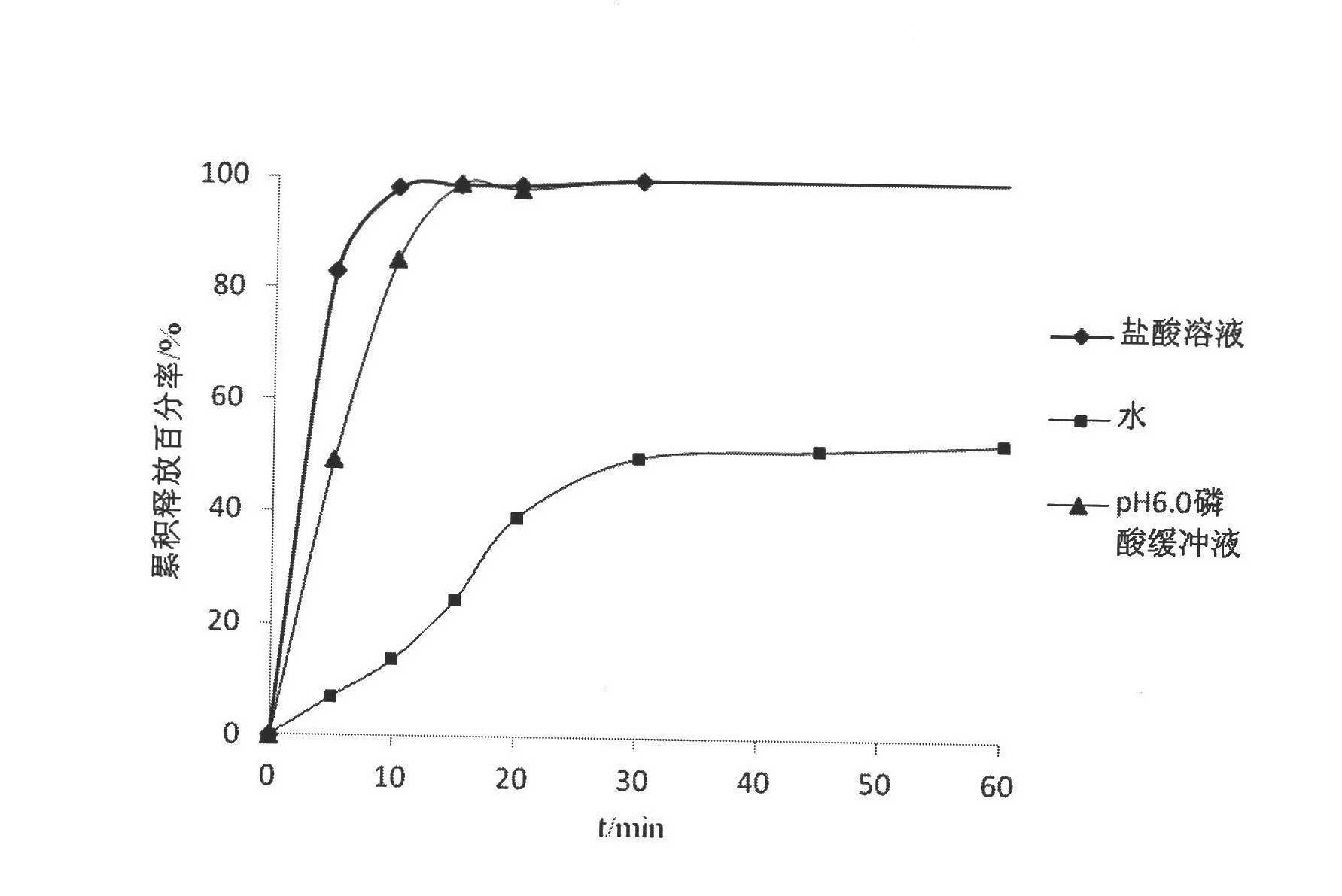
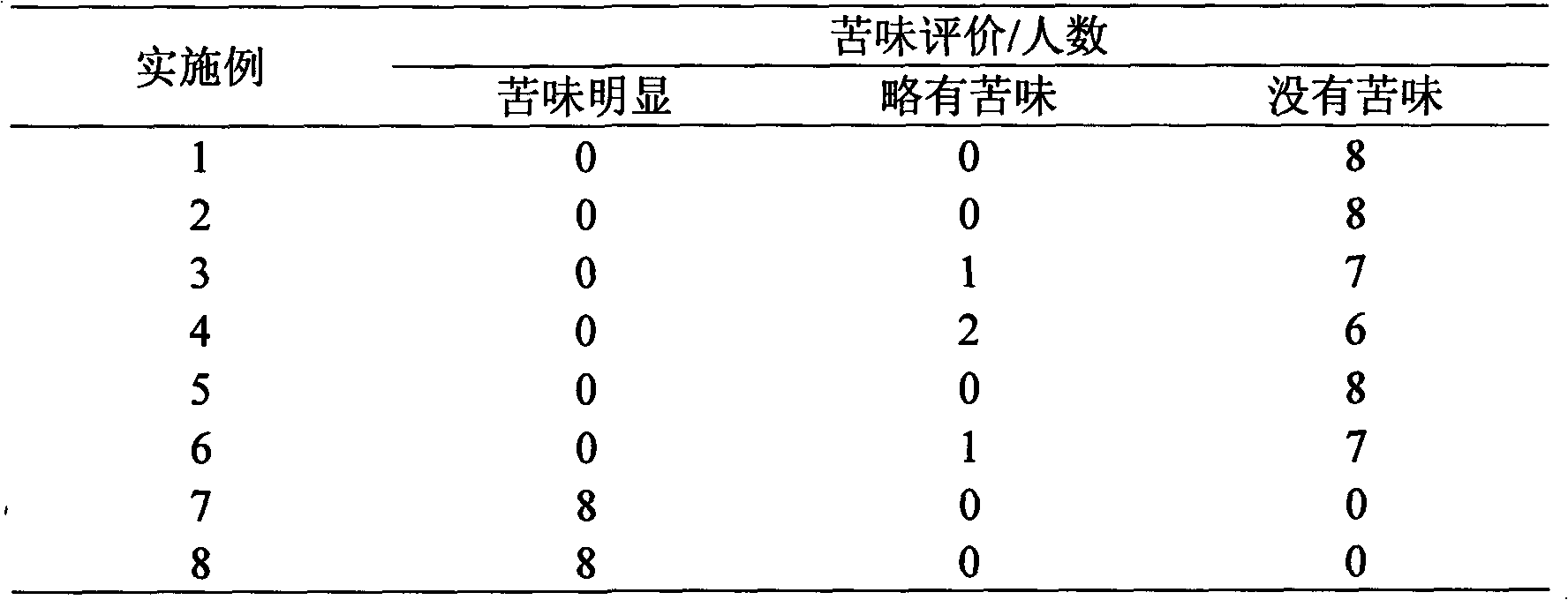
Image
Examples
Embodiment 1
[0039] 1. Prescription of spiramycin particles:
[0040] The proportion of the spiramycin microparticles in solid weight percentage is as follows: 50% spiramycin, 22% stearyl alcohol, 12% microcrystalline cellulose, and 16% ethyl cellulose.
[0041] 2. The preparation process of spiramycin particles:
[0042] Mix spiramycin with stearyl alcohol, microcrystalline cellulose, and ethyl cellulose, use water as a binder to prepare soft materials, use extrusion spheronization to prepare particles, extrude with a sieve plate with an aperture of 0.25mm, and use 1000rpm to rotate Make a round. The obtained microparticles were first dried in an oven at 40°C for 2 hours to remove moisture, and then heat-treated at 70°C for 0.5 hour. After cooling to room temperature, size the particles and collect particles with a particle size between 0.05 and 0.3 mm.
[0043] The above-mentioned spiramycin particles are mixed with auxiliary materials such as suspending agent, corrective agent, essen...
Embodiment 2
[0045] 1. Prescription of spiramycin particles:
[0046] The proportion of the spiramycin microparticles in solid weight percentage is as follows: 30% of spiramycin, 10% of glyceryl distearate, 15% of microcrystalline cellulose, and 35% of acrylic resin.
[0047] 2. The preparation process of spiramycin particles:
[0048] Mix evenly the microcrystalline cellulose and acrylic resin with half the prescription quantity, put it in the centrifugal granulator, adjust the position of the mixing knife, make the material in the vortex reflux state in the granulator, adjust the spray gun Atomization pressure, jet flow and blast flow. Use water as a binder, control the process conditions until the mother nucleus grows to an average particle size of 0.05-0.15mm, and then take it out. Dry at 60°C to remove moisture. Sieve and select particles with a particle size between 0.05 and 0.15 mm as the core and place them in a centrifugal granulator. Mix spiramycin, glyceryl distearate, and t...
Embodiment 3
[0050] 1. Prescription of spiramycin particles:
[0051] The proportion of the spiramycin microparticles in solid weight percentage is as follows: 49% spiramycin, 35% glyceryl behenate, 10% microcrystalline cellulose, and 6% starch.
[0052] 2. The preparation process of spiramycin particles:
[0053] Using a method known in the pharmaceutical industry, mix the prescribed amount of spiramycin, glyceryl behenate and microcrystalline cellulose evenly and place them in the fluidized bed cavity to adjust the air intake to keep the materials in a suitable fluidized state. The wind temperature is 45°C, adjust the atomization pressure and spray speed of the spray, and use the prepared 5% starch slurry as the binder to granulate. After the prescribed amount of slurry is completely sprayed, continue to dry at an inlet air temperature of 45°C. After the material is completely dried, set the inlet air temperature to 80°C and heat-treat for 0.5 hours. Stop heating, continue blasting, a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com