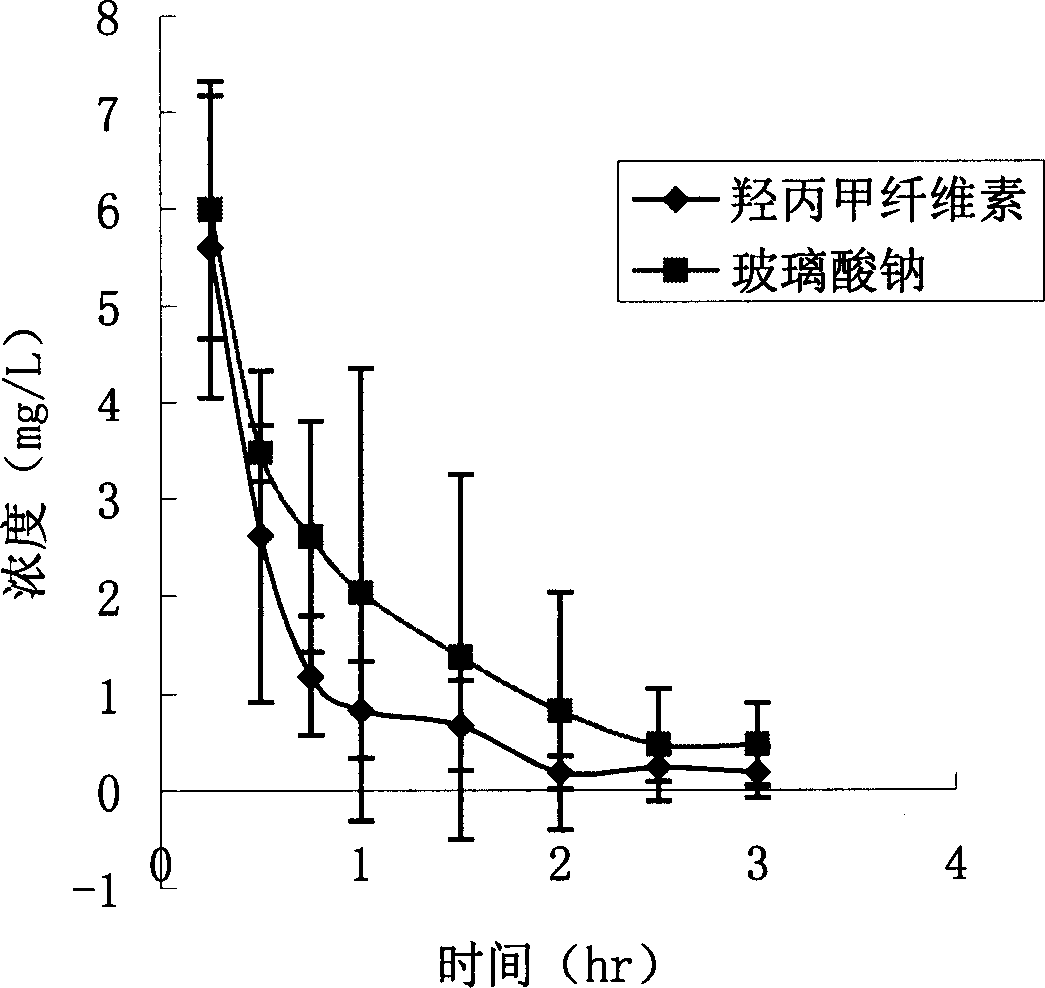
Macrolide antibiotics sodium hyaluronate eye transfer system
A macrolide and antibiotic technology, applied in the field of macrolide antibiotic ophthalmic delivery system, can solve the problems of limiting the application of MA drugs, difficult eyelid blinking, limited synergistic effect and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Prescription: (based on 100ml eye drops)
[0057] Azithromycin 1g
[0058] Sodium Hyaluronate (SH) 1g
[0059] Hydrochloric acid amount
[0060] Sodium chloride appropriate amount
[0061] Benzalkonium Chloride 0.005g
[0062] Appropriate amount of sodium hydroxide
[0063] Add water for injection to 100ml
[0064] Process: Dissolve 1g of sodium hyaluronate in about 40ml of water for injection. Take an appropriate amount of hydrochloric acid to dissolve azithromycin, adjust the pH to 6.0-7.0 with sodium hydroxide, add benzalkonium chloride and sodium chloride therein, and stir evenly. Mix the two solutions together, add water for injection to 100ml, and stir well. Sterilize by filtration with a 0.25um membrane, pack in aliquots, and obtain.
Embodiment 2
[0066] Prescription: (based on 100ml eye drops)
[0067] Clarithromycin 1g
[0068] Sodium Hyaluronate (SH) 1g
[0069] Hydrochloric acid amount
[0070] Sodium chloride appropriate amount
[0071] Benzalkonium Chloride 0.005g
[0072] Appropriate amount of sodium hydroxide
[0073] Add water for injection to 100ml
[0074] Process: Dissolve 1g of sodium hyaluronate in about 40ml of water for injection. Take an appropriate amount of hydrochloric acid to dissolve clarithromycin, adjust the pH to 6.0-7.0 with sodium hydroxide, add benzalkonium chloride and sodium chloride therein, and stir evenly. Mix the two solutions together, add water for injection to 100ml, and stir well. Sterilize by filtration with a 0.25um membrane, pack in aliquots, and obtain.
Embodiment 3
[0076] Prescription: (based on 100ml eye drops)
[0077] Erythromycin 1g
[0078] Sodium Hyaluronate (SH) 1g
[0079] Hydrochloric acid amount
[0080] Sodium chloride appropriate amount
[0081] Benzalkonium Chloride 0.005g
[0082] Appropriate amount of sodium hydroxide
[0083] Add water for injection to 100ml
[0084] Process: Dissolve 1g of sodium hyaluronate in about 40ml of water for injection. Take an appropriate amount of hydrochloric acid to dissolve erythromycin, adjust the pH to 6.0-7.0 with sodium hydroxide, add benzalkonium chloride and sodium chloride therein, and stir evenly. Mix the two solutions together, add water for injection to 100ml, and stir well. Sterilize by filtration with a 0.25um membrane, pack in aliquots, and obtain.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com