Method for detecting initial material II in apixaban through reversed-phase high performance liquid chromatography
A reversed-phase high-performance liquid chromatography and chromatographic detection technology is applied in the field of reversed-phase high-performance liquid chromatography to detect the starting material II of apixaban, which can solve the problems of cumbersome operation, fast peak output, long system equilibration time, etc. Accurate test results, enhanced control, and simple composition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] 1. Determine the detection wavelength
[0040] Accurately weigh an appropriate amount of starting material II, and use mobile phase (acetonitrile-0.02mol / L potassium dihydrogen phosphate = 45:55) to prepare a solution with a concentration of 20 μg / ml as the test solution.
[0041] Take the above-mentioned test solution and scan it in the wavelength range of 200nm to 400nm. The test results are shown in Table 1.
[0042] The ultraviolet scanning result of the present invention of table 1
[0043] project
Peak wavelength(nm)
the peak
Valley wavelength(nm)
valley
225.2
0.5711
241.8
0.0210
[0044] The test results show that the detection wavelength in the range of 220nm-230nm is suitable for the HPLC detection method of the present invention; preferably, the detection wavelength is 225±2nm.
[0045] 2. High performance liquid chromatography detection method
[0046] Detection by reversed-phase high-pe...
Embodiment 2
[0146] Embodiment 2 adopts reverse-phase high-performance liquid chromatography to detect:
[0147] Filler of the chromatographic column: the model of the chromatographic column is WatersSymmetryC18, the specifications are: the inner diameter is 4.6mm, the length is 150mm, and the particle size of the filler is 5μm;
[0148] Mobile phase: acetonitrile-0.02mol / L potassium dihydrogen phosphate (volume ratio 45:55);
[0149] Column temperature: 35°C;
[0150] Flow rate: 1.0ml / min;
[0151] Detection wavelength: 225nm;
[0152] Injection volume: 20 μl.
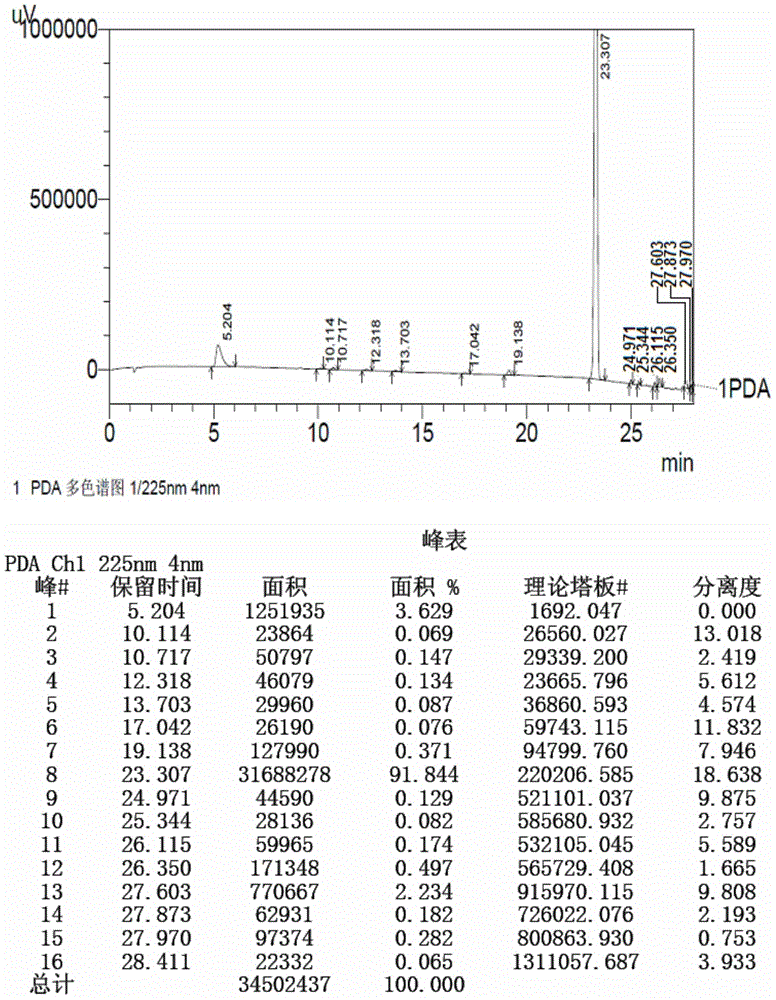
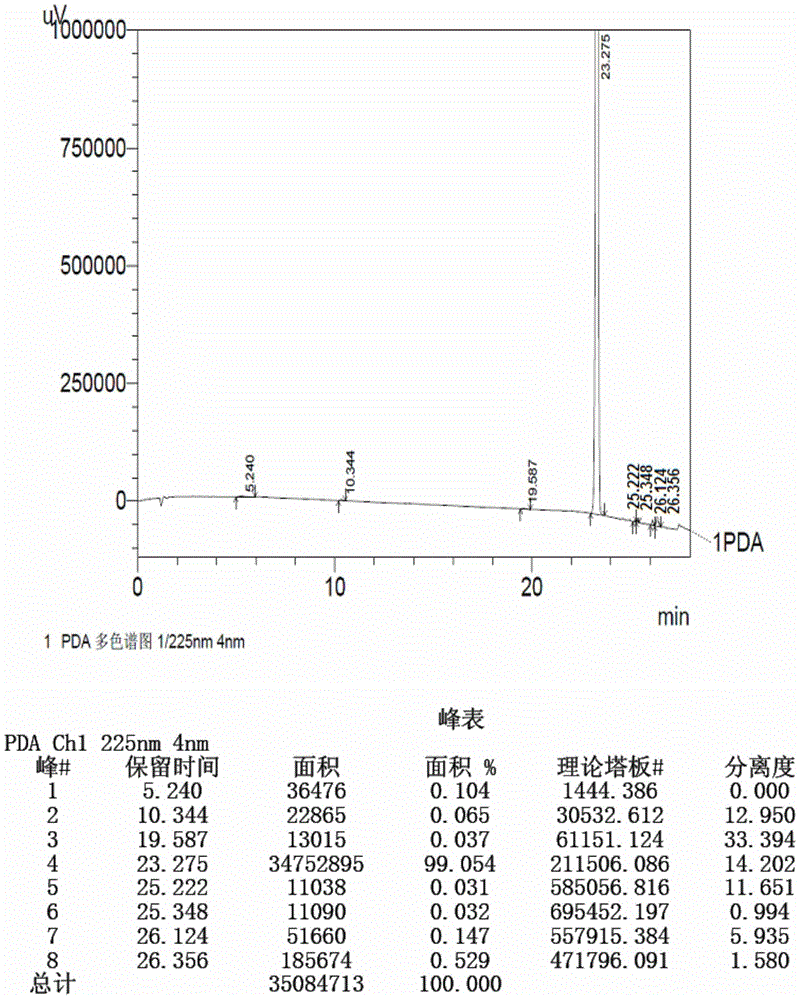
[0153] Prepare test solution according to the method of Example 1 and sample injection measurement, the result shows: starting material II (retention time is 23.253min) and the degree of separation of adjacent impurity is 52.578, theoretical plate number (calculated by starting material II ) is 144820, and the peak purity index of the starting material II is 1.000000. The reversed-phase high performance liquid chromatography o...
Embodiment 3
[0154] Embodiment 3 adopts reverse-phase high performance liquid chromatography to detect:
[0155] Filler of the chromatographic column: the model of the chromatographic column is WatersSymmetryC18, the specifications are: the inner diameter is 4.6mm, the length is 150mm, and the particle size of the filler is 5μm;
[0156] Mobile phase: acetonitrile-0.02mol / L potassium dihydrogen phosphate (volume ratio is 40:60);
[0157] Column temperature: 35°C;
[0158] Flow rate: 1.0ml / min;
[0159] Detection wavelength: 225nm;
[0160] Injection volume: 20 μl. .
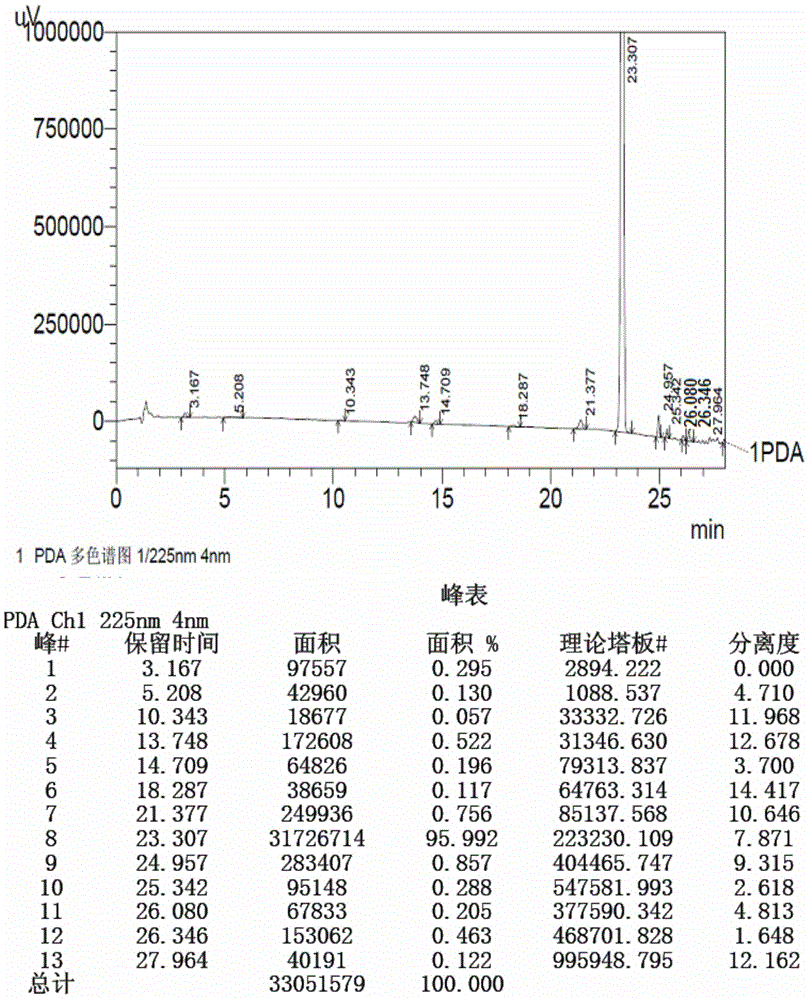
[0161] Prepare test solution according to the method of Example 1 and sample injection measurement, the result shows: starting material II (retention time is 28.612min) and the degree of separation of adjacent impurities is 66.003, theoretical plate number (calculated by starting material II ) is 15827, and the peak purity index of the starting material II is 1.000000. The reversed-phase high performance liquid chromatog...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The inside diameter of | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com