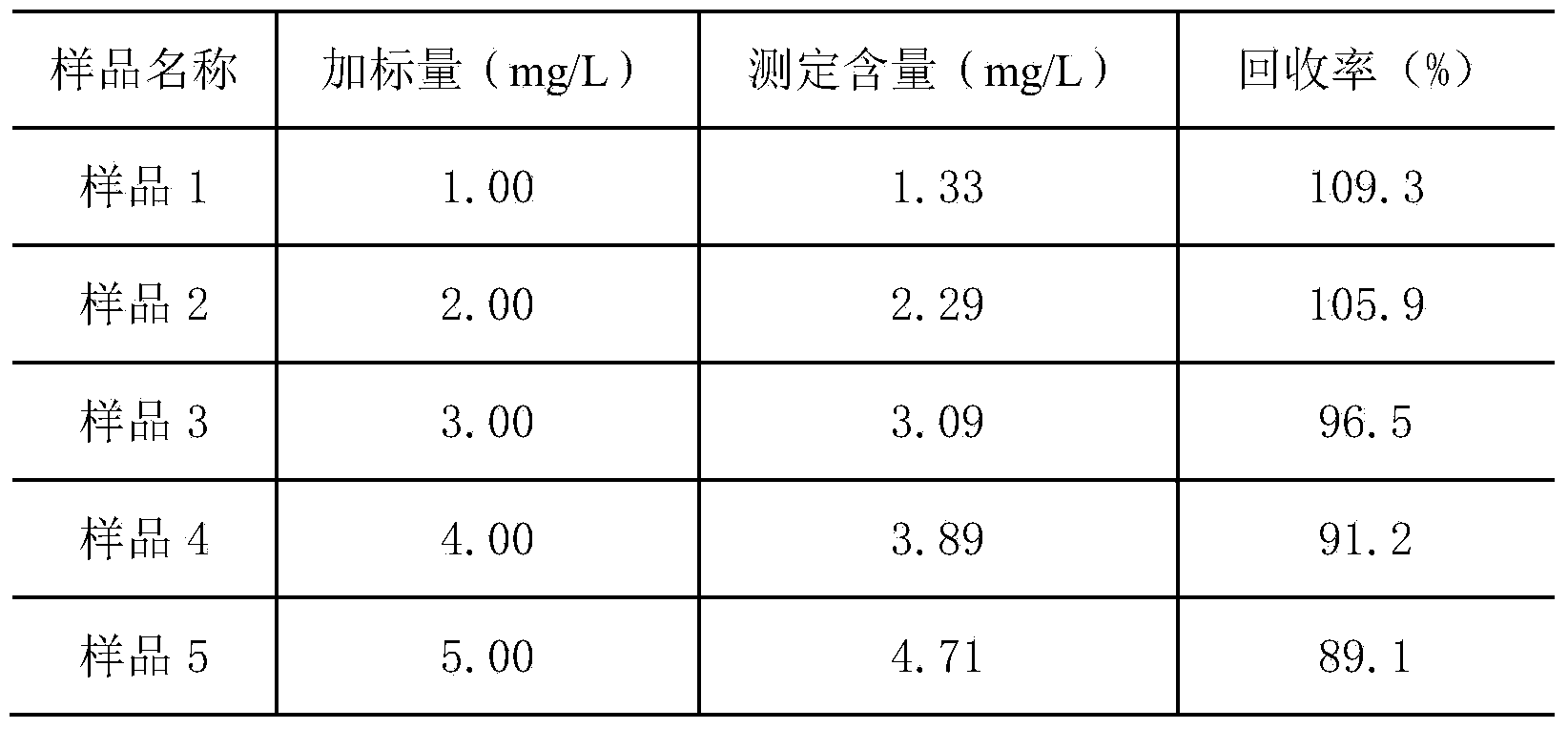
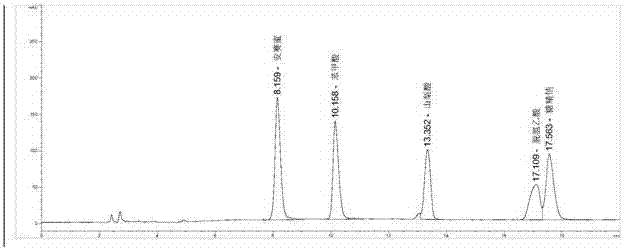
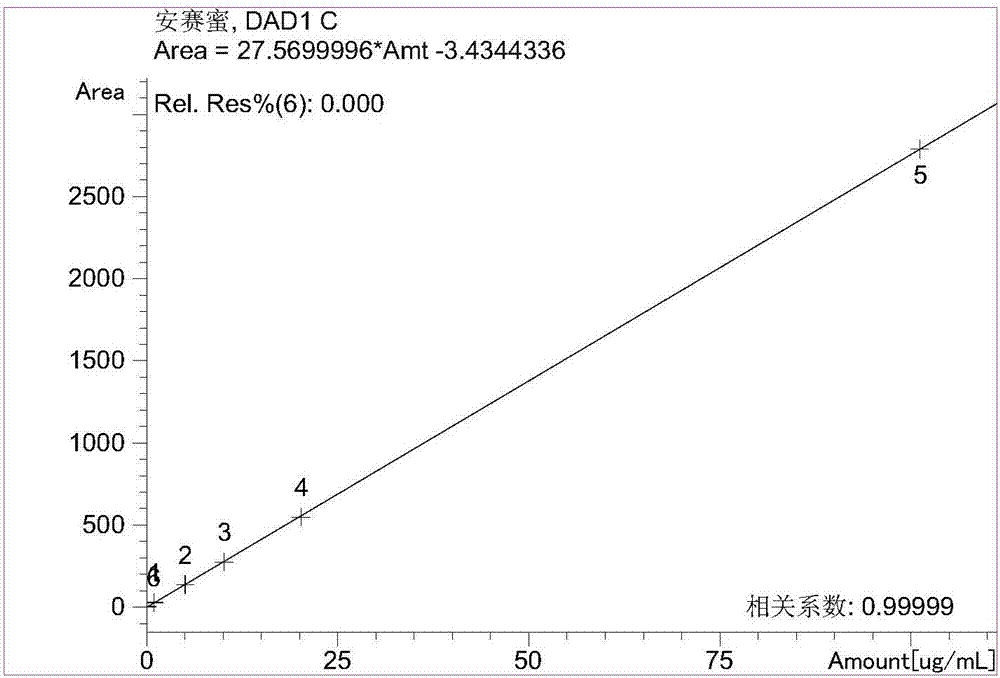
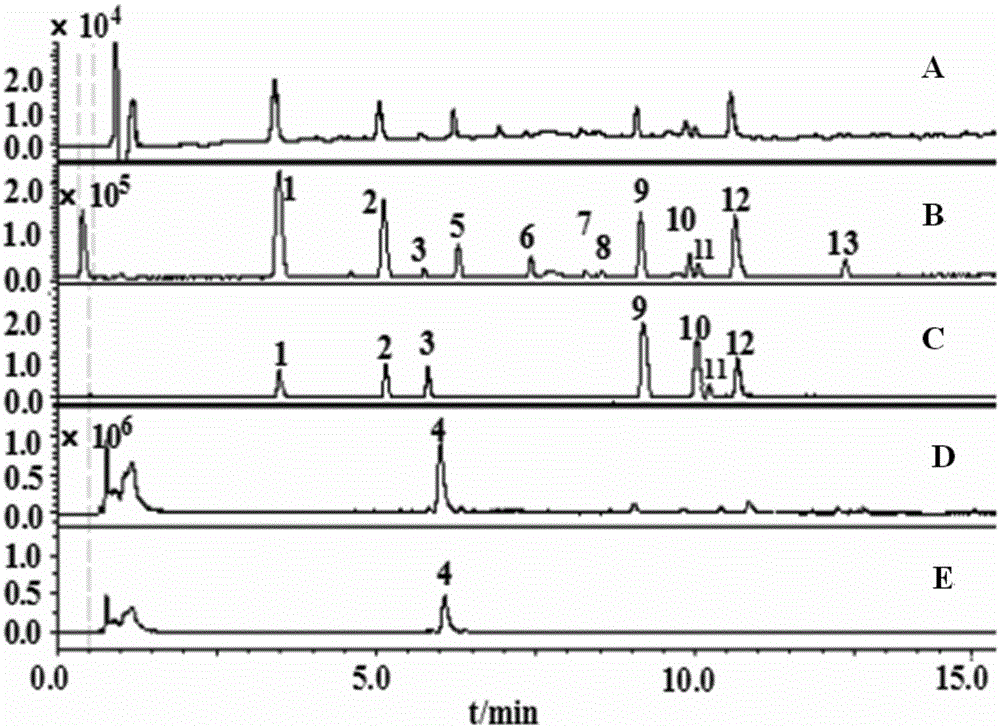
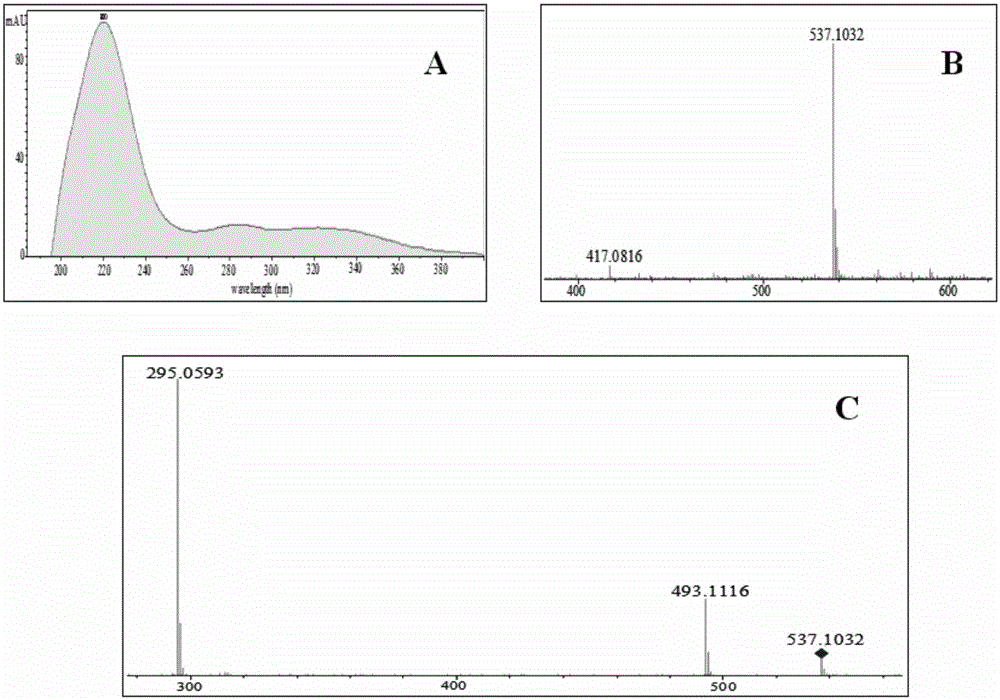
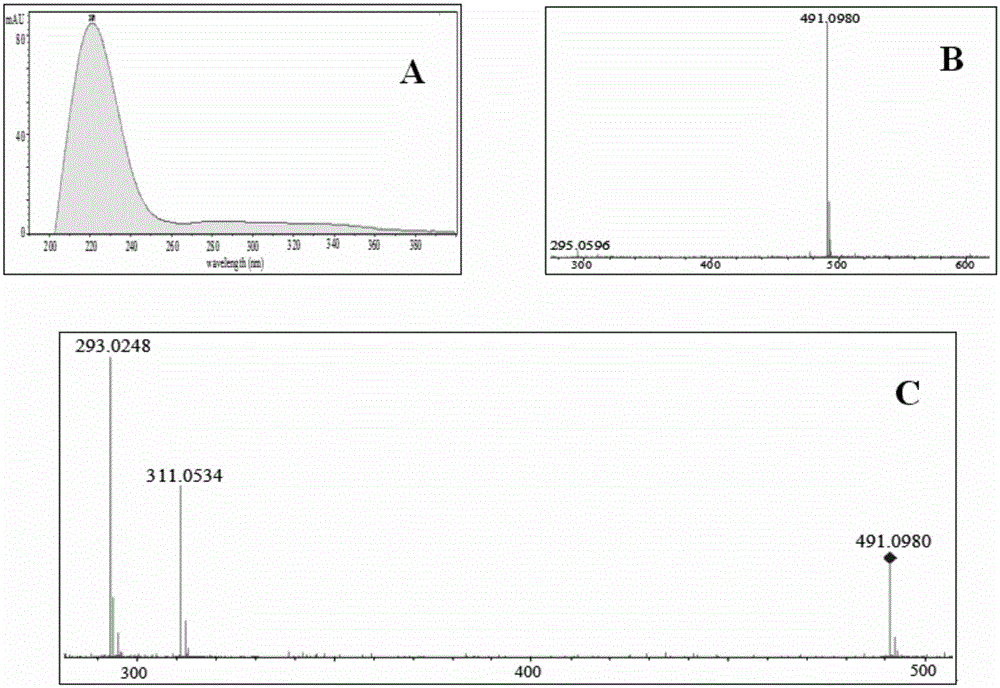
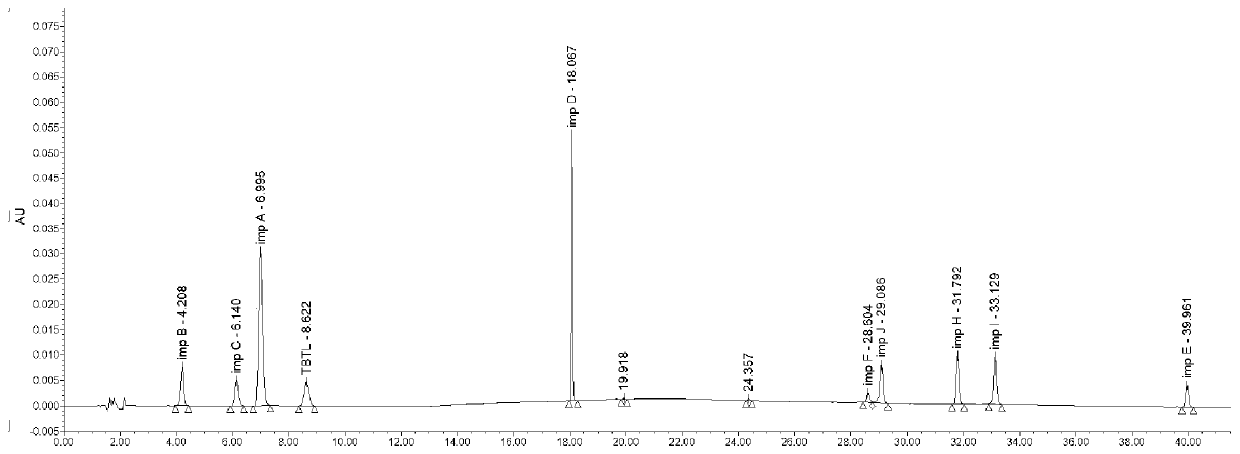
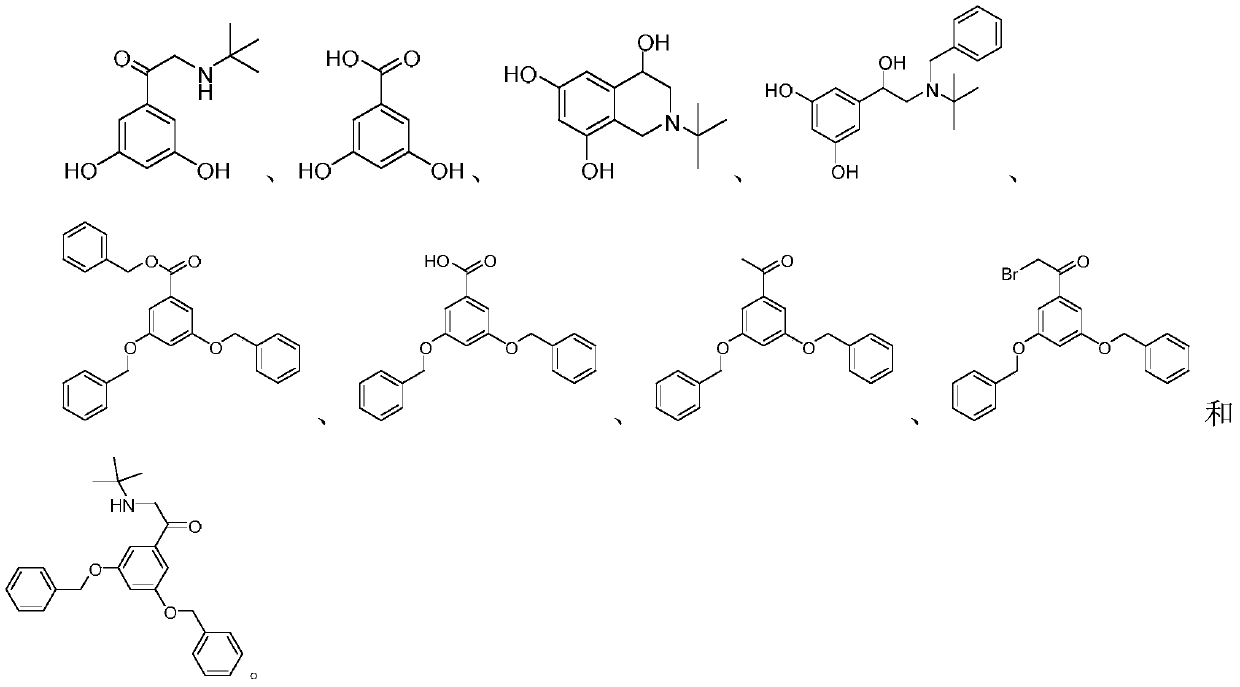
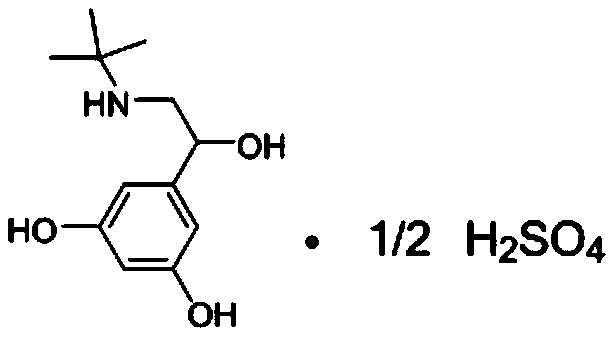
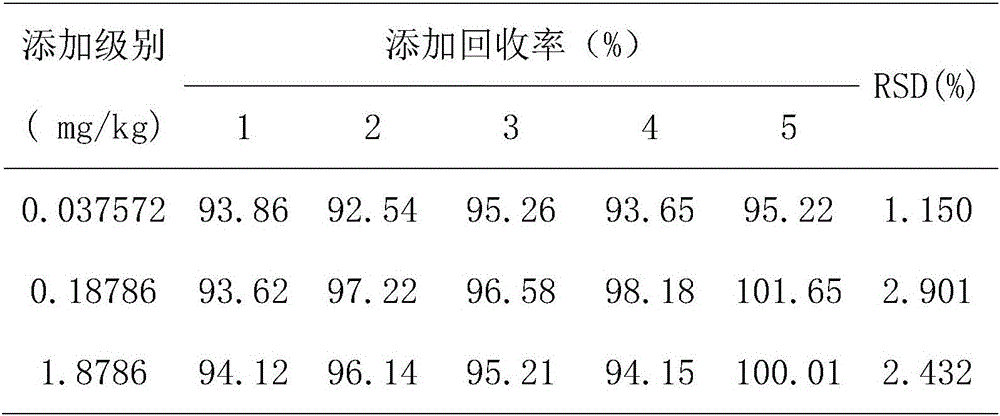
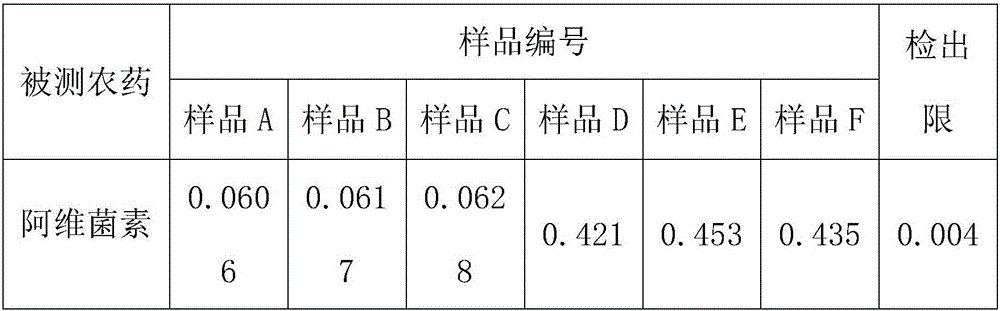
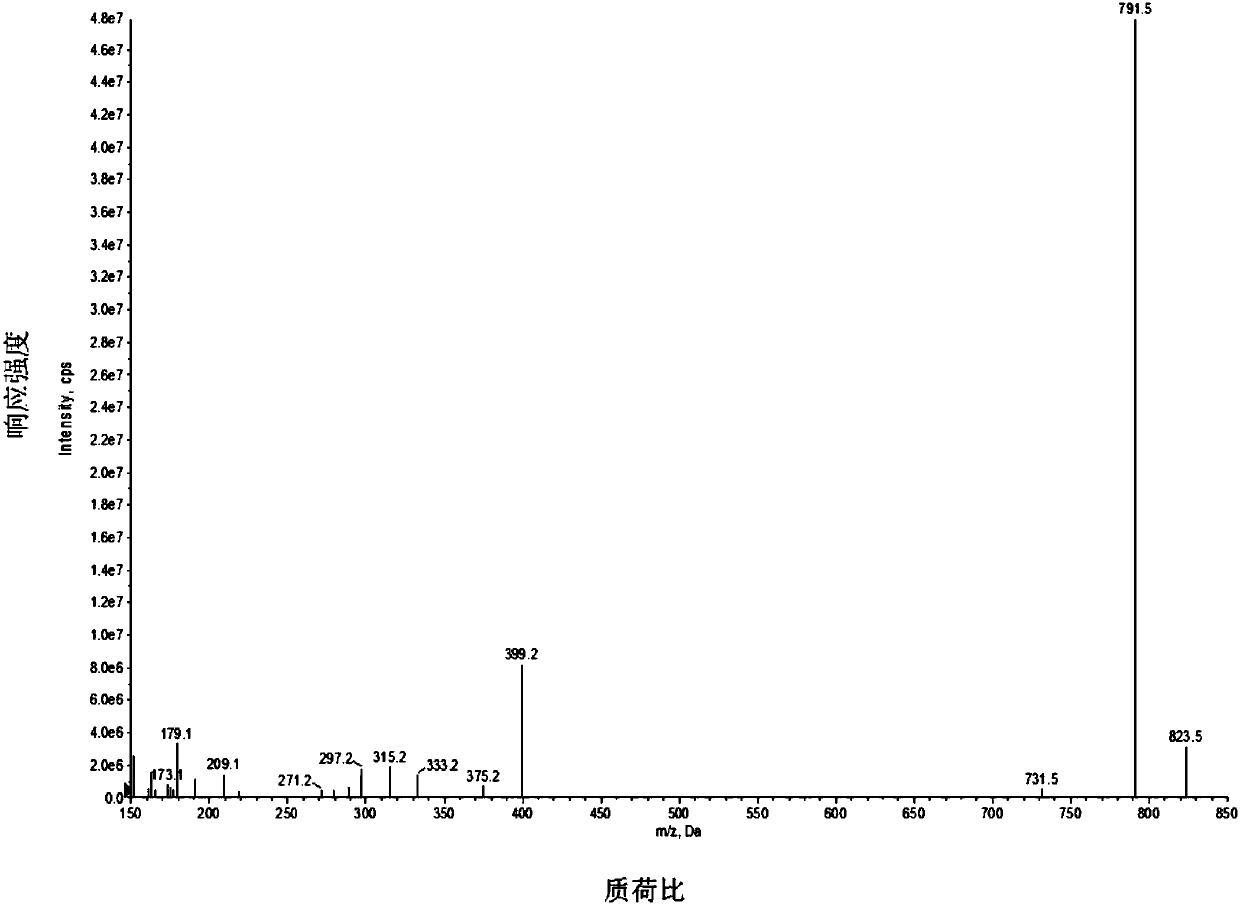
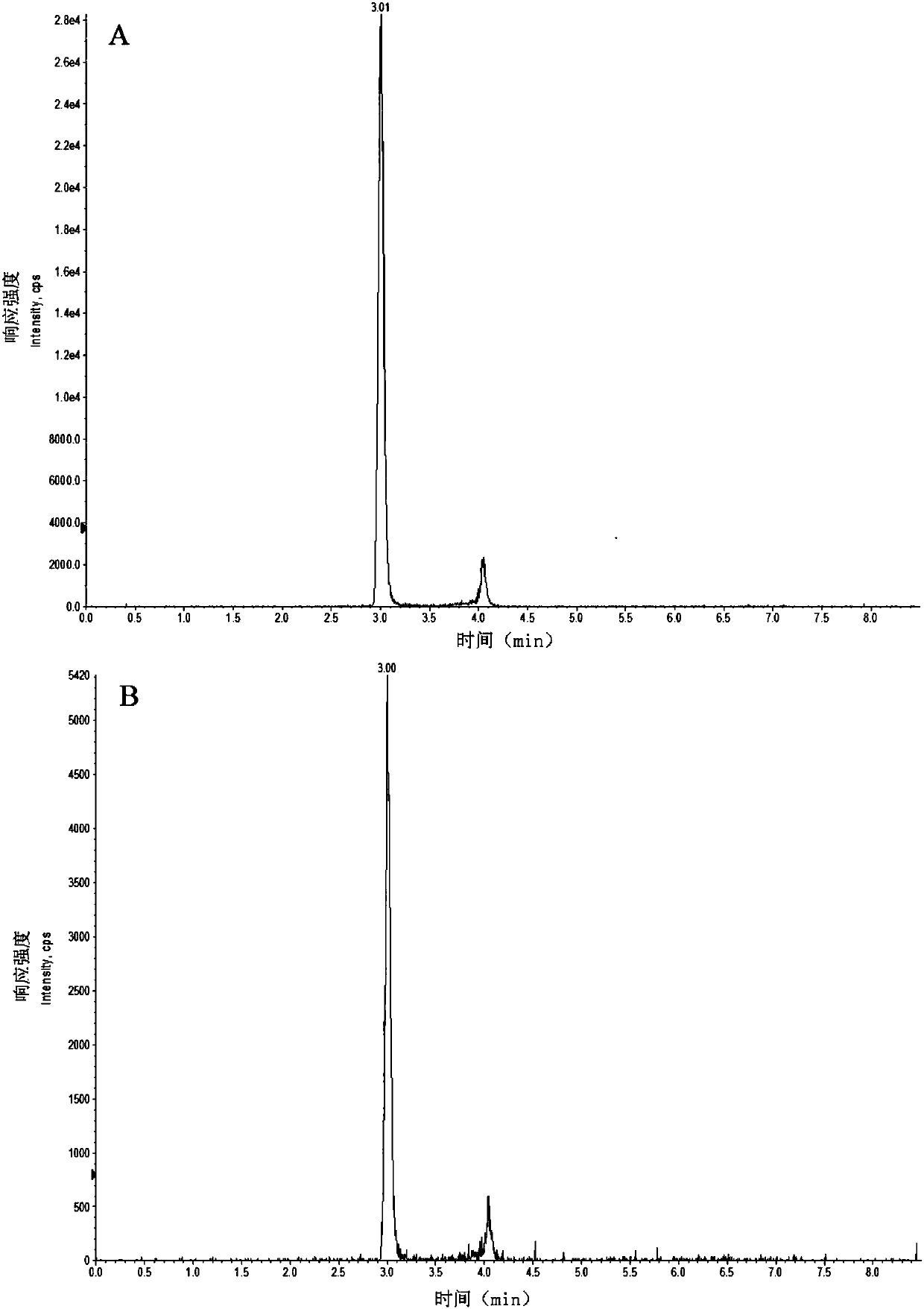
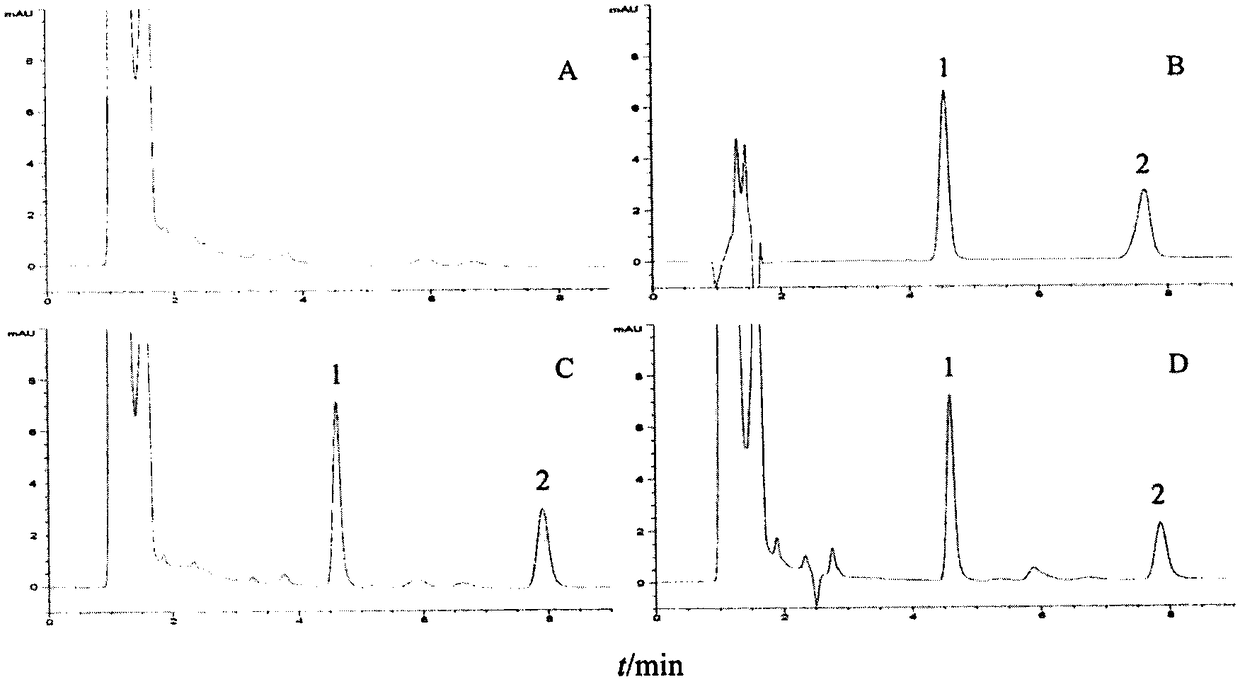
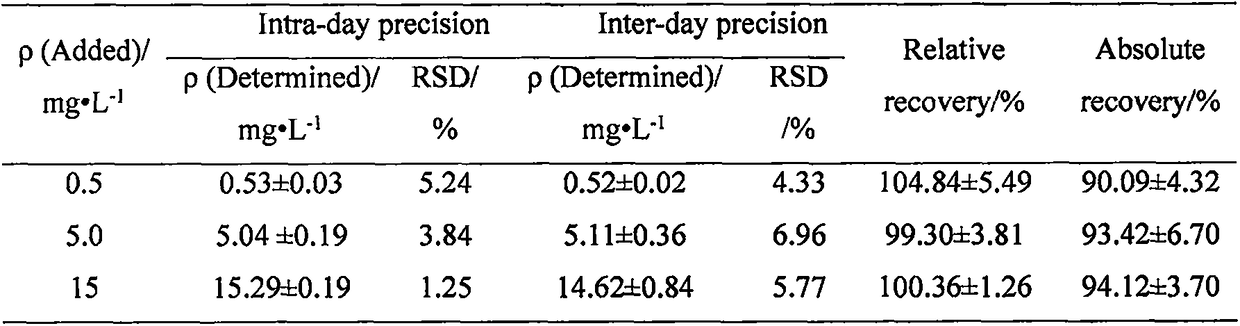
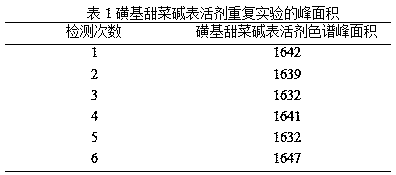
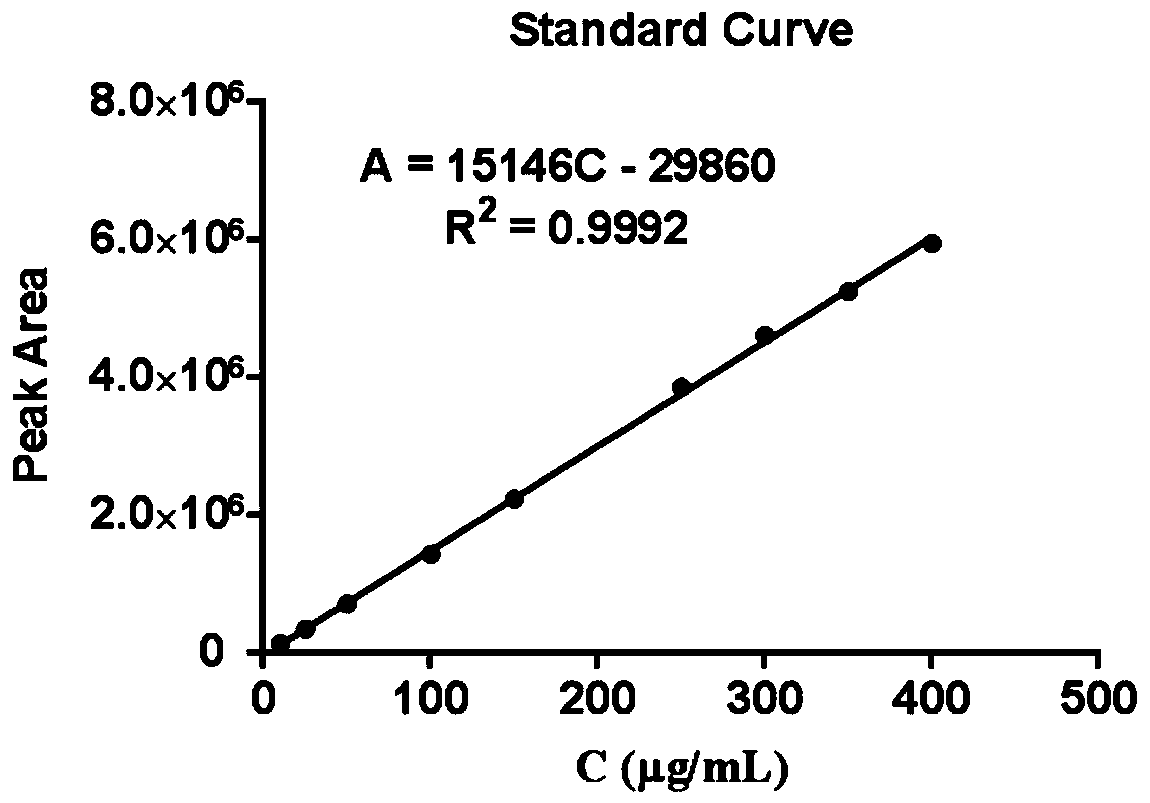
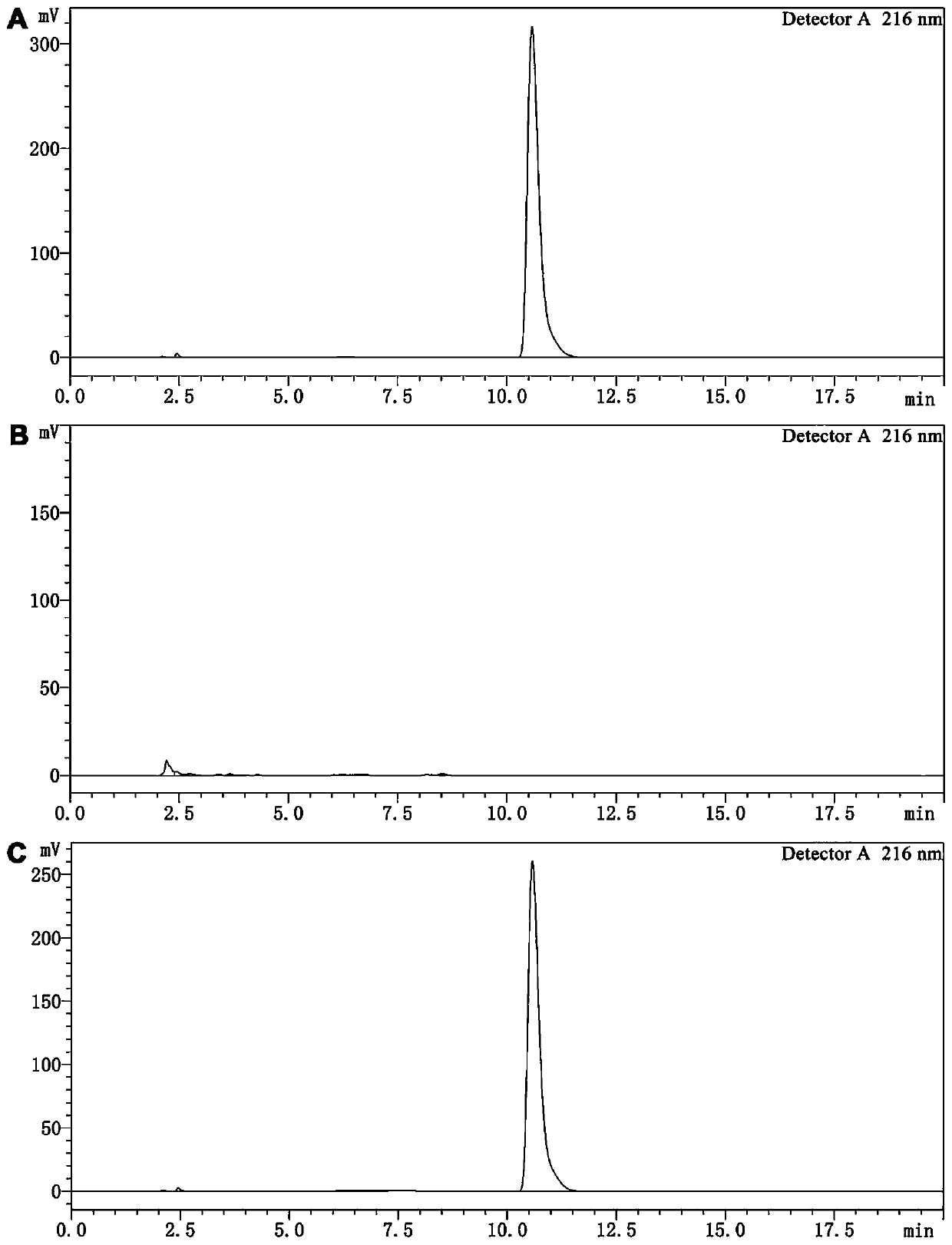
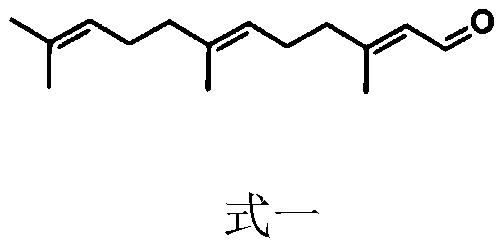
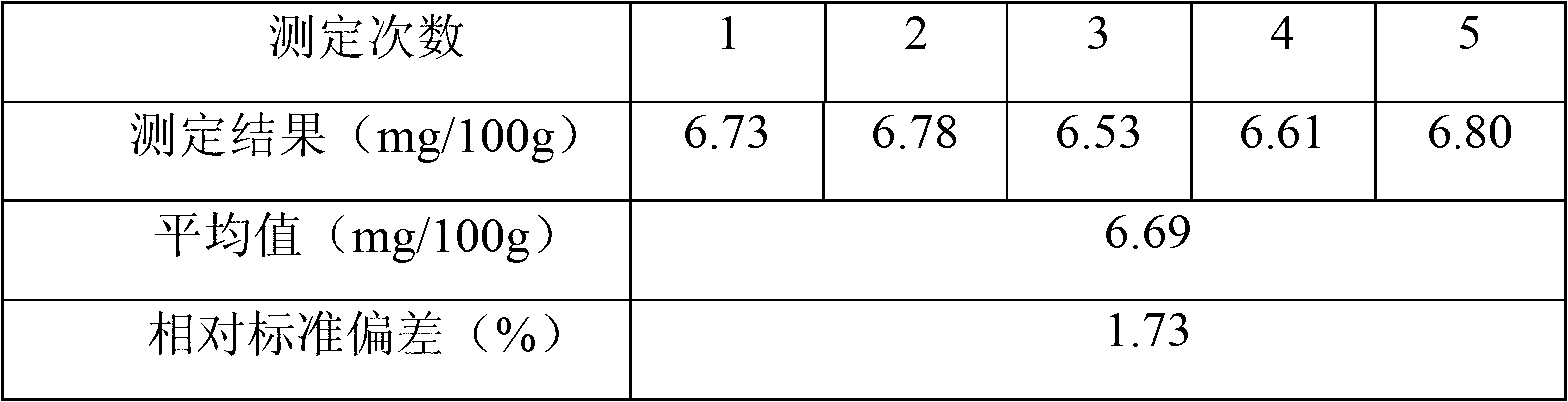
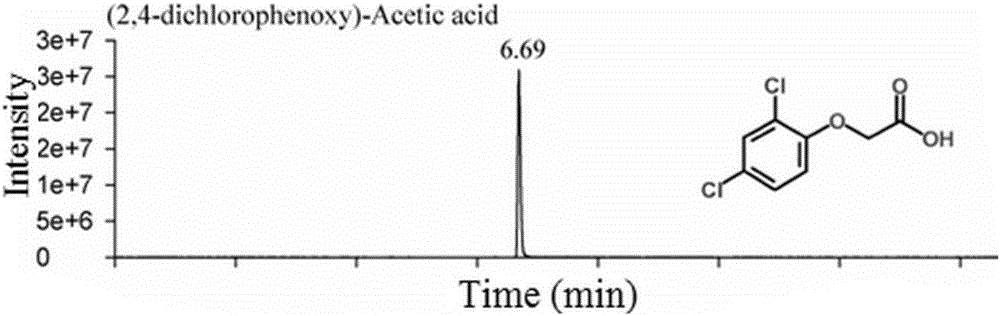
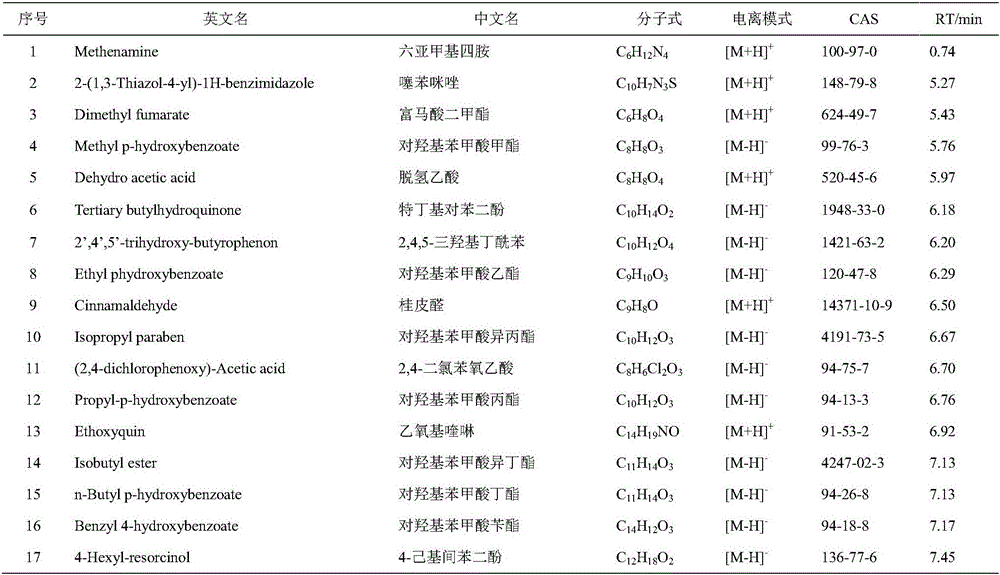
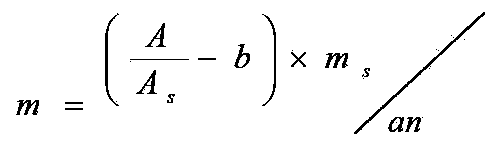Patents
Literature
62 results about "High-performance Liquid Chromatography-UV" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
An analytical technique where high performance liquid chromatography with an ultraviolet light detector is used to separate, identify, and quantify substances in a sample.
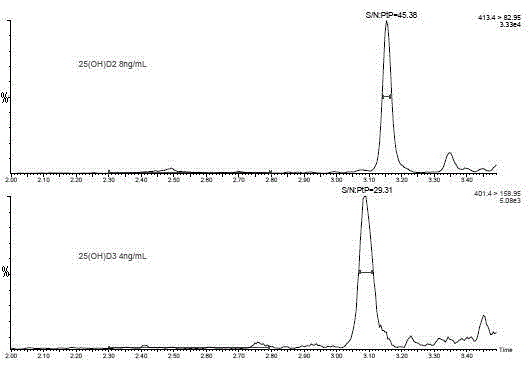
Method for detecting 25-hydroxy-vitamin D through ultra-performance liquid chromatography-tandem mass spectrometry
The invention provides a method for detecting 25-hydroxy-vitamin D through a high-sensitivity high-throughput ultra-performance liquid chromatography-tandem mass spectrometry device. The method comprises adding an internal standard substance of 25-hydroxy-vitamin D into a serum sample, adding a zinc sulfate solution and a methanol solution into the mixture so that protein deposition is realized, carrying out full mixing, adding a n-hexane solvent into the mixture, carrying out extraction, carrying out full mixing, carrying out centrifugation, taking the supernatant, carrying out nitrogen blowing drying, adding a compounded solution into the supernatant to obtain a sample to be detected, detecting the sample to be detected through an ultra-performance liquid chromatography-tandem quadrupole mass spectrometry device and carrying out quantitation through an internal standard curve method based on 25-hydroxy-vitamin D2 and / or 25-hydroxy-vitamin D3 quantitative and qualitative ion pair retention time as qualitative basis. The method has advantages of simple pre-treatment process, good specificity, good matrix interference resistance, short detection time, high throughput, high detection precision, high sensitivity and low cost.
Owner:袁洪
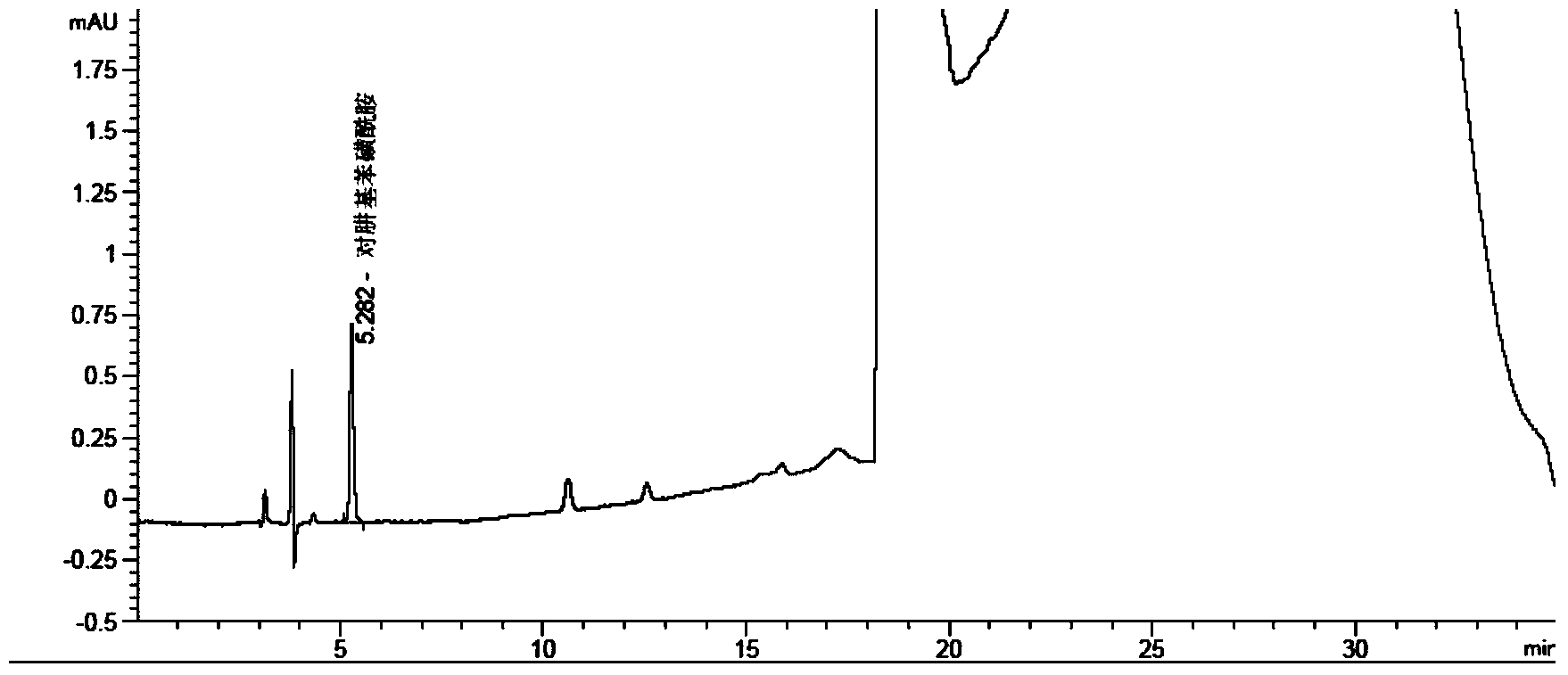
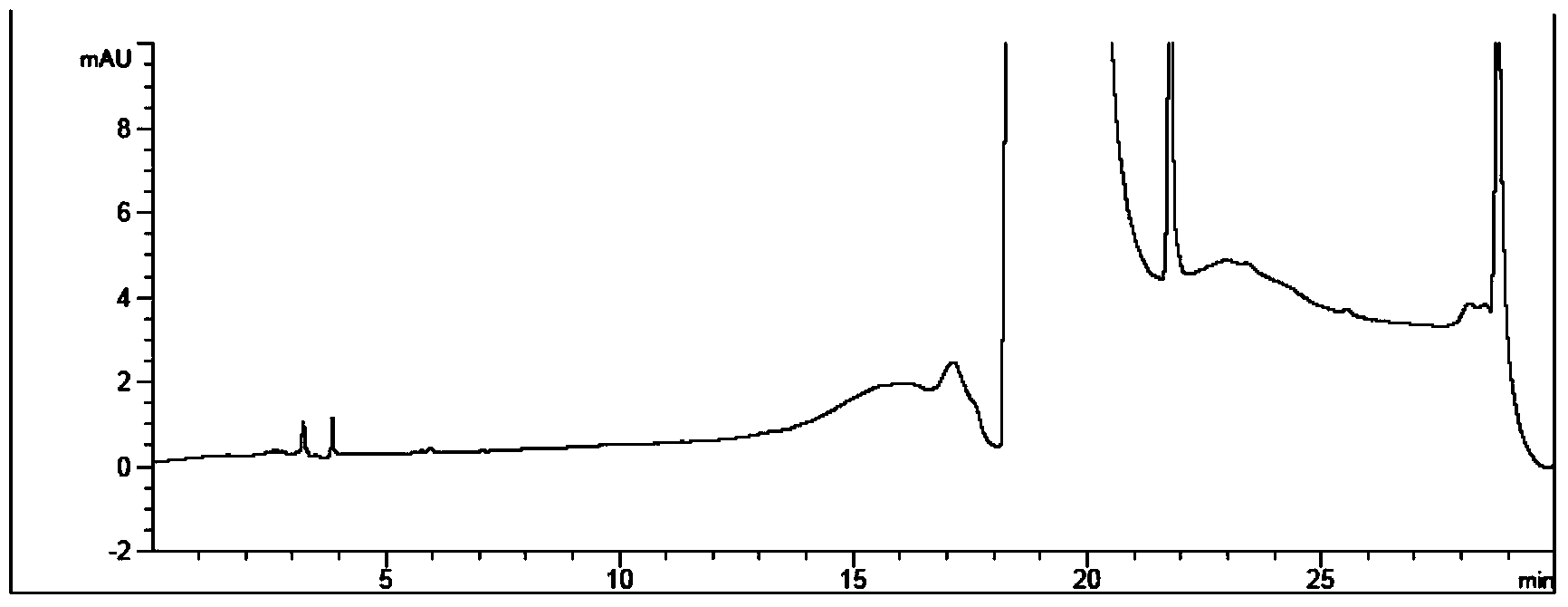
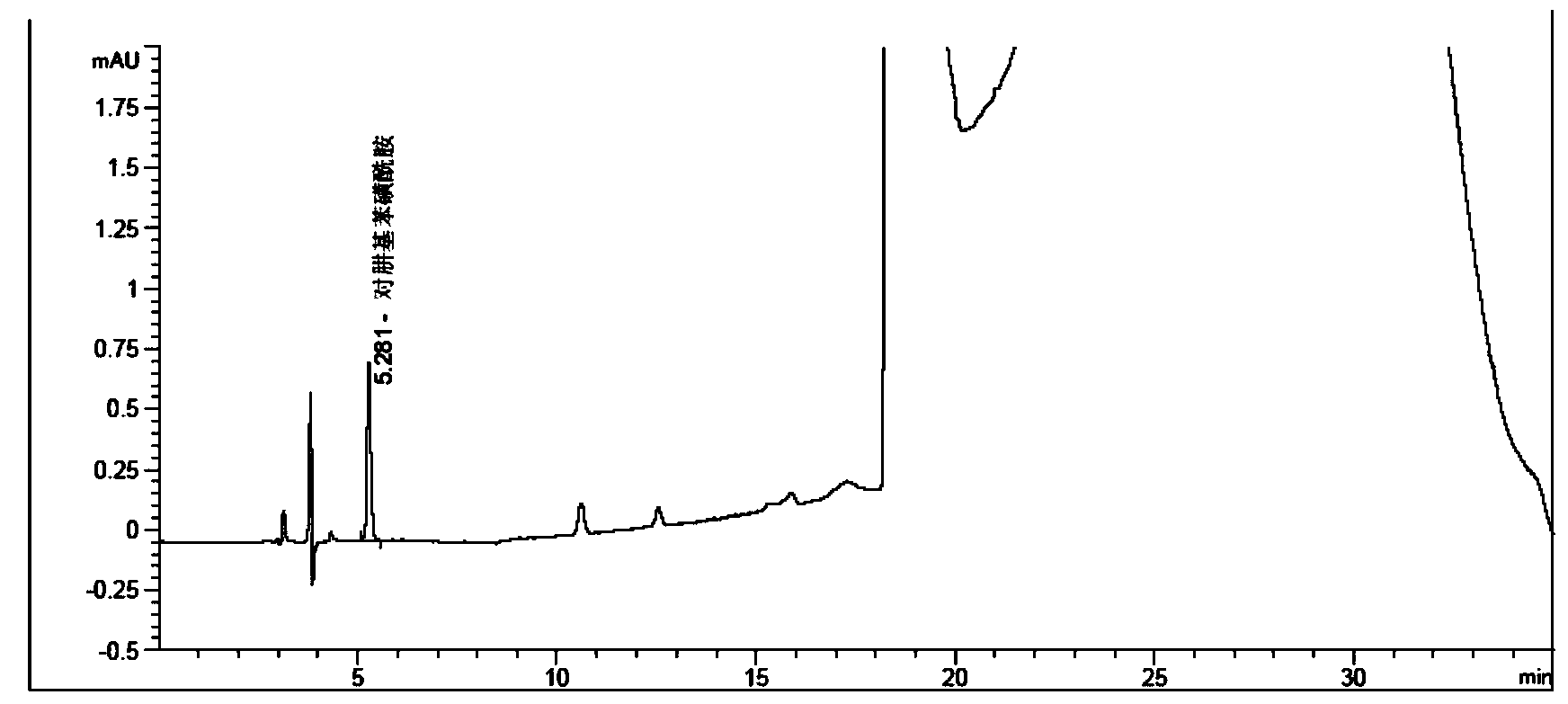
Method for measuring phenylhydrazine compound residues in crude drugs through HPLC (high performance liquid chromatography)
The invention discloses a method for measuring genotoxic impurities (or doubtful genotoxicity), namely phenylhydrazine compound residues, in crude drugs through the HPLC (high performance liquid chromatography). The detection is directly implemented by taking phenyl bonded silica gel as a chromatographic column of a solid phase and organic phase and buffer solution mixed solvent gradient elution as a mobile phase. The detection method is high in detection sensitivity, strong in specificity, high in precision, high in accuracy, convenient to operate and strong in adaptability and can be used for detecting phenylhydrazine compounds in various crude drugs, and the quality of the crude drugs can be effectively controlled.
Owner:WATERSTONE PHARMA WUHAN
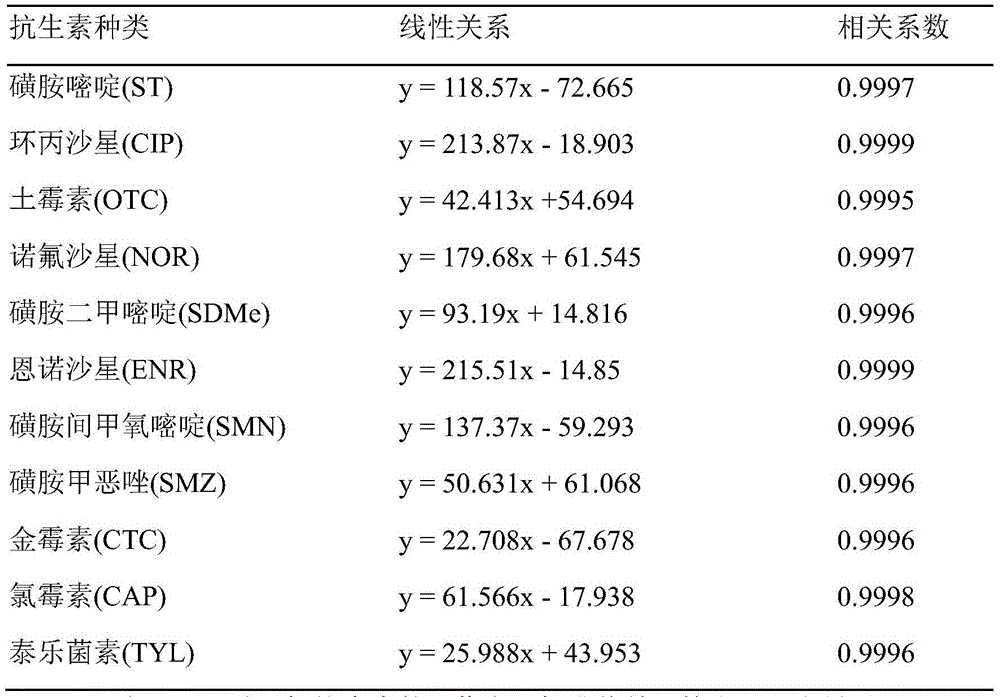
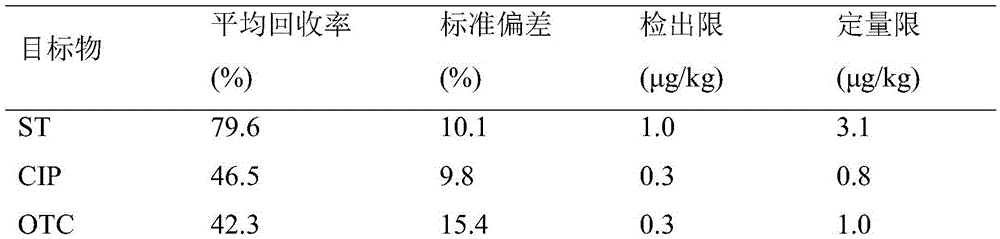
Method for simultaneously detecting various antibiotics in livestock and poultry manure by high performance liquid chromatography
ActiveCN105548392APromote absorptionDoes not affect qualitativeComponent separationFecesAntibiotic Y
The invention relates to an antibiotic detection method, and in particular, relates to a method for simultaneously detecting various antibiotics in livestock and poultry manure by high performance liquid chromatography; the method comprises the steps: pretreating a livestock and poultry manure sample, to obtain a to-be-detected liquid; performing analyte separation of the to-be-detected liquid by a high performance liquid chromatograph, and calculating peak areas corresponding to separated residual antibiotics; and quantitatively calculating according to a standard curve, to obtain the residual content. The method is applied in detection of 11 kinds of residual antibiotics in the livestock and poultry manure; compared with a high performance liquid chromatography-mass spectrometry coupled technique, the method has the advantages of lower detection cost and high detection efficiency, can simultaneously separate 11 kinds of antibiotics, has analytes without interference of major interferents, and basically does not affect qualitative and quantitative analysis of the analytes.
Owner:INST OF AGRI RESOURCES & REGIONAL PLANNING CHINESE ACADEMY OF AGRI SCI
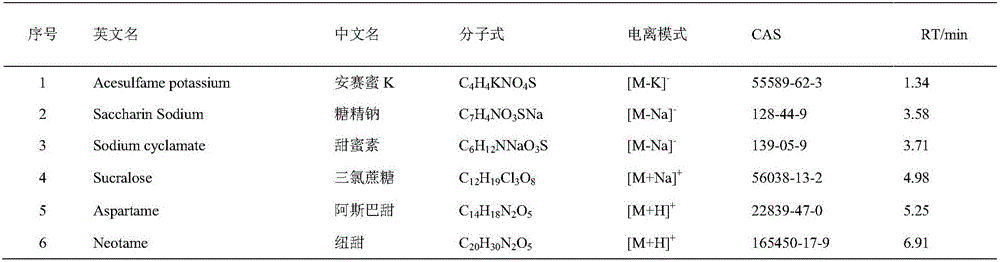
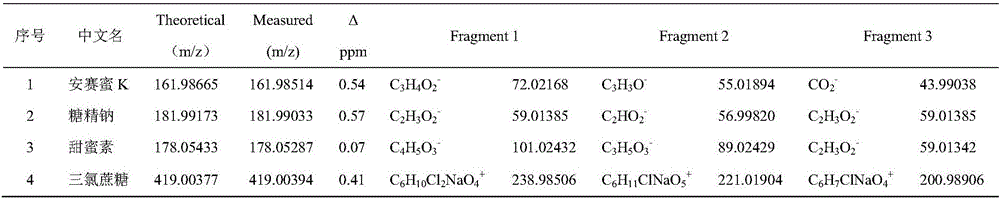
Method for screening sweetening agent in milk and dairy products by applying ultra-high performance liquid chromatography-quadrupole electrostatic field orbit ion trap mass spectrometry
InactiveCN106290693AEasy to operateFast positive and negative switchingComponent separationScreening methodQuadrupole
The invention discloses a method for screening a sweetening agent in milk and dairy products by applying ultra-high performance liquid chromatography-quadrupole electrostatic field orbit ion trap mass spectrometry, and belongs to the technical field of food safety inspection. An optimized QuEChERS method is used for preprocessing samples; the ultra-high performance liquid chromatography-quadrupole electrostatic field orbit ion trap mass spectrometry is applied for performing screening, a scanning mode of full-scanning / data-dependency collection and full-scanning / variable data-independent collection and full-scanning / full-ion fragmentation collection is adopted, a positive ion or negative ion mode is selected for scanning, and complete first-order and second-order spectra are obtained; Extract Finder 2.5 software is applied for processing the obtained first-order and second-order spectra, and information of a compound is extracted. Information in a built spectrum library and a tested object screening result are compared, and the composition of the sweetening agent in the milk and dairy products is ensured. An adopted mass spectrometer has the main advantages that the resolution is high, qualitative and quantitative results are accurate, sensitivity is high, and quality precision is high and can be applied to complex matrix analysis. The established screening method has the good application prospect in the field of food.
Owner:SHAANXI UNIV OF SCI & TECH
High-performance liquid chromatography method for measuring nucleotide in milk powder
ActiveCN102680601AThe separation method is simpleEasy to separateComponent separationHydrogen NitrateFood additive
The invention relates to a high-performance liquid chromatography method for measuring nucleotide in milk powder, and belongs to the technical field of detection for food additives. The method comprises the following steps of: preparing a contrast solution by using a nucleotide standard substance; weighing a test sample, dissolving the test sample in distilled water through ultrasonic oscillation, so that the nucleotide in the milk powder is dissolved in the distilled water; adjusting the pH value of the test sample solution by hydrogen nitrate until the pH value is 3 to 4, precipitating proteins, filtering and removing the proteins, fixing the volume of a filtrate by using water to obtain a to-be-tested solution; measuring nucleotide chromatographic peak areas in the test solution and the contrast solution by high performance liquid chromatography; and calculating the nucleotide content in the test sample by an external standard method according to the concentration of the nucleotidein the contrast solution, the nucleotide chromatographic peak area in the test solution and the mass of the test sample. The method is simple, convenient to operate and high in precision; proteins can be completely separated, so that interference with the measurement for the nucleotide is eliminated; and a good condition is supplied to measurement of the nucleotide in the test sample.
Owner:SHANDONG KAISHENG NEW MATERIALS
Method for detecting initial material II in apixaban through reversed-phase high performance liquid chromatography
ActiveCN105044269AEfficient separationPurpose of enhanced controlComponent separationHigh-performance Liquid Chromatography-UVImpurity
The invention discloses a method for detecting an initial material II in apixaban through reversed-phase high performance liquid chromatography. The method comprises the following steps: 1, preparing a sample solution; 2, detecting the sample through reversed-phase high performance liquid chromatography; and 3, calculating the content of every impurity and the content of total impurities in the sample through an area normalization technique. The method for detecting initial material II related substances in apixaban through reversed-phase high performance liquid chromatography realizes complete separation of the chromatographic peaks of the initial material II and all the impurities through a simple mobile phase component; and the detection result of the method is accurate and reliable, so the method makes control of the quality of the initial material in the apixaban synthesis process possible.
Owner:CHENGDU BAIYU PHARMA CO LTD
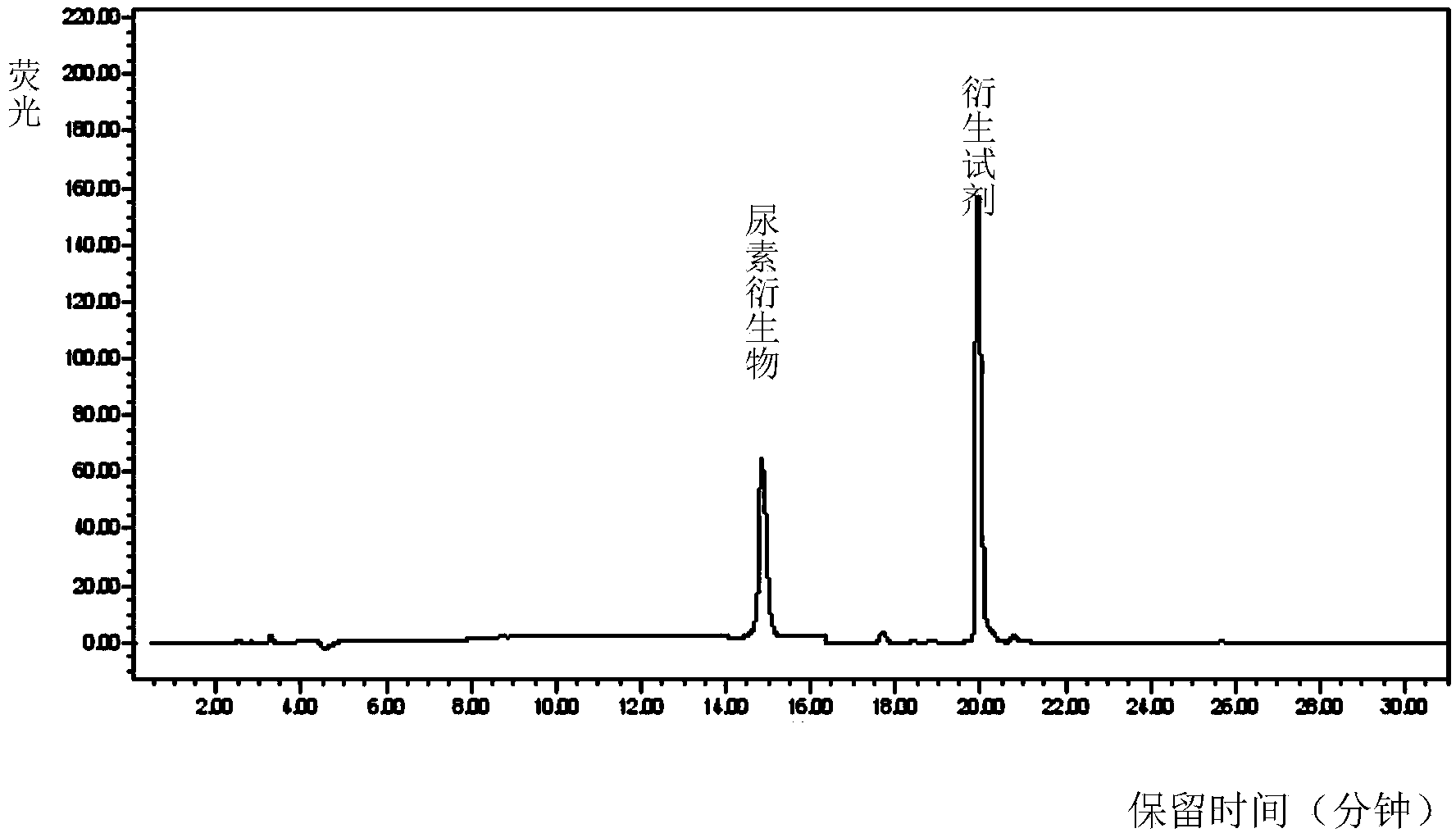
Method for detecting content of urea in brewed wine by using high performance liquid chromatography
InactiveCN103760266AThe pretreatment method is simpleEasy to handleComponent separationUrea derivativesFluorescence
The invention relates to a method for detecting the content of urea in brewed wine by using high performance liquid chromatography. The method comprises the following steps: weighing an appropriate sample, selecting a proper solvent, and performing pre-treatment on the sample by using precolumn derivatization, rotary evaporation concentration and the like; and detecting urea derivatives by using a high performance liquid chromatograph connected with a fluorescence detector, wherein the organic flow phase and the inorganic flow phase are in variable ratio, the flow speed is set as 1mL / min, the sampling amount is set as 10 microliters, the chromatographic column is selected as the C18 chromatographic column, the column temperature is 40-50 DEG C, and the detection wavelength of the fluorescence detector as follows: the lambda ex is 208nm, and the lambda em is 308nm. The detection method provided by the invention has the characteristics of being simple, fast and high in recycling rate.
Owner:CENT TESTING INT GRP CO LTD
Method of determining low-content paricalcitol through high performance liquid chromatography-tandem mass spectrometry method and application thereof
InactiveCN105372340AStrong specificityHigh sensitivityComponent separationInternal standardDissolution
The invention belongs to the technical field of analytical chemistry and particularly relates to a method of determining low-content paricalcitol through a high performance liquid chromatography-tandem mass spectrometry method and application thereof. According to the method, the high performance liquid chromatography-tandem mass spectrometry method is adopted for determination, an internal standard method is also adopted, and dutasteride is used as an internal standard substance. The method is high in specificity, sensitivity and accuracy, the detection limit of the method can reach 40 pg / ml, and the quantization limit can reach 80 pg / ml. The method can be widely applied to testing of the content and dissolution rate of a low-content paricalcitol preparation, and particularly testing of the dissolution rate of a paricalcitol soft capsule.
Owner:CHONGQING HUAPONT PHARMA
Method used for detecting caffeic acid content in cigarette sidestream smoke by high performance liquid chromatography-tandem mass spectrum
ActiveCN103399118AImprove qualitative accuracyHigh sensitivityComponent separationCaffeic acidTotal particulate matter
The invention discloses a method used for detecting caffeic acid content in cigarette sidestream smoke by high performance liquid chromatography-tandem mass spectrum. The method comprises following steps: (1) total particulate matters in smoke are collected by a fishtail cover and a filter disc; (2) the filter disc is placed in a methanol solution containing internal standard substances for extraction so as to obtain an extract; (3) the extract is filtered, and then is detected by a high performance liquid chromatography-tandem mass spectrometer; and (4) the content of caffeic acid is quantitatively analyzed by internal standard method. According to the method used for detecting caffeic acid content in cigarette sidestream smoke by high performance liquid chromatography-tandem mass spectrum, pre-treatment is simple, separating effect is excellent, qualitative analysis is accurate, sample analysis time is short, and detection sensitivity is high.
Owner:CHINA TOBACCO JIANGSU INDAL
Method for testing food additives in foods by virtue of high performance liquid chromatography
InactiveCN107167532AImprove detection efficiencyDetection contentComponent separationFood additiveRetention time
The invention belongs to the technical field of food detection methods and particularly relates to a method for testing food additives in foods by virtue of a high performance liquid chromatography. The method comprises the followings steps: preparing standard series working solutions; preparing to-be-tested liquid; and determining: testing the standard series working solutions and the to-be-tested liquid by virtue of a high performance liquid chromatograph with a DAD detector, carrying out gradient elution, and carrying out detection under a variable wavelength, wherein a flow phase of the high performance liquid chromatograph is gradient-mixed liquid of an ammonium acetate solution and methanol, and an acetic acid solution is added into the ammonium acetate solution. By improving a sample processing method, five additives can be synchronously extracted, and the detection efficiency is improved; by optimizing the ratio and analysis parameters of the flow phase of the high performance liquid chromatography, chromatographic peaks of the five additives are well separated, and the accurate quantitative analysis is facilitated; and by detecting the area of the chromatographic peak corresponding to the characteristic absorption wavelength of each additive in a retention time, a relatively low detection limit can be obtained, and the interference of other components can be well eliminated.
Owner:广东省中鼎检测技术有限公司
Chemical component identification and active component screening method of Shenxiong glucose injection
The invention provides a chemical component identification and active component screening method of a Shenxiong glucose injection and belongs to the technical field of traditional Chinese preparation quality controlling. In the method, main chemical components in the Shenxiong glucose injection are subjected to comprehensive on-line analysis and identification through ultra-high performance liquid chromatography-diode array detector-in-series qudropole time-of-flight mass spectrometry (UPLC-DAD-Q / TOF) to determine the structure of the compound; and meanwhile, active components in the Shenxiong glucose injection are subjected to screening with combination of myocardial cell extraction technology with ultra-high performance liquid chromatography-electrospray ionization-triple qudropole tandem mass spectrum (UPLC-ESI-MS / MS). The method can quickly and effectively determine the chemical structures of the components in traditional Chinese medicine and the main active components of the traditional Chinese medicine, and provides scientific experiment evidence of quality evaluation of the Shenxiong glucose injection.
Owner:GUIZHOU JINGFENG INJECTION
Method for analyzing related substances of terbutaline sulfate via high performance liquid chromatography
ActiveCN110057932AImprove detection efficiencyHigh sensitivityComponent separationGradient elutionHigh-performance Liquid Chromatography-UV
The invention provides a method for analyzing related substances of terbutaline sulfate via high performance liquid chromatography. A C18 reversed chromatographic column is used, a mobile phase is a mixture of a 0.05 M ammonium acetate buffer solution with pH 4.0 and methanol, gradient elution is carried out, and qualitative and quantitative detection of terbutaline and 9 impurities is achieved byonce sample injection. Compared with the prior art, the method provided by the invention has the advantages of being able to not only detect more impurities, but also having good sensitivity to various impurities. In the specific application, according to the detection result of each batch of samples, the limit of the key impurity formulated, and the economic applicability in the actual production process is greatly improved.
Owner:SHANGHAI XUDONG HAIPU PHARMA
High performance liquid chromatography detection method for abamectin content in edible vegetable oil
InactiveCN106226444AEfficient extractionLow costComponent separationLiquid Chromatography-FluorescencePretreatment method
The invention discloses a high performance liquid chromatography detection method for the abamectin content in edible vegetable oil. The method comprises the steps that a sample is subjected to vortex oscillation extraction through methanol, purified with an ODS C18 solid-phase extraction column, subjected to derivatization through N-methylimidazole and trifluoroacetic anhydride and lastly determined through a liquid chromatography-fluorescence method. According to the method, few reagents are adopted, and the cost is reduced; a sample pretreatment method is simple, the purification effect is good, maintenance of instruments in the using process is reduced, and the technical problems that when a liquid chromatography-ultraviolet detector is used, the sensitivity is too low, and matrix interference is serious are solved; a determined result is accurate and good in repeatability, the detection specificity and sensitivity are high, and a reference is provided for detection of the abamectin content in the edible vegetable oil.
Owner:FOSHAN HAIYUE ZHIDA TECH CO LTD
Rapid ultra-high performance liquid chromatography-mass spectrum testing method for rifampicin residues in aquatic products
InactiveCN107941980AGuaranteed stabilityAvoid detection impactComponent separationIsotopeConcentration gradient
The invention discloses a rapid ultra-high performance liquid chromatography-mass spectrum testing method for rifampicin residues in aquatic products. The method comprises the following steps: extracting a sample, purifying the sample, preparing a standard rifampicin concentration gradient working solution with a rifampicin stable isotope interior label; testing the standard rifampicin concentration gradient working solution with the stable isotope interior label of rifampicin and a sample liquid to be tested under identical chromatography and mass spectrum conditions in sequence; acquiring quantitative ion pair and qualitative ion pair information of rifampicin in a multi-reaction monitoring modes (MRM) of tandem quadrupole mass spectrometry; by taking a peak area ratio of rifampicin quantitative ion pairs to rifampicin stable isotope interior label standard substance ion pairs extracted through multi-reaction monitoring as a longitudinal coordinate, and a mass concentration of rifampicin as a transverse coordinate, performing linear regression analysis, introducing the peak area ratio of sample rifampicin to an interior label standard substance into a linear equation, thereby obtaining the content of a target substance in a sample.
Owner:ZHEJIANG FISHERIES TECH EXTENSION STATION
Ultra-high performance liquid chromatography method for determining content of rheum lhasaense
ActiveCN103713067AReduce analysis and detection timeReduce dosageComponent separationPhosphoric acidGradient elution
The invention relates to the field of medicament analysis, and discloses an ultra-high performance liquid chromatography method for determining the content of rheum lhasaense. The method comprises the steps of: by taking acetonitrile-0.1% phosphoric acid water solution as a mobile phase, gradiently eluting rheum lhasaense-carried sample solution to enter an ultra-high performance liquid chromatograph; carrying out gradient elution by taking the acetonitrile-0.1% phosphoric acid water solution as a mobile phase; setting a standard sample, determining the response values of deoxyrhaponticin peak, triptophenolide peak, and polydatin peak in the rheum lhasaense sample by using an ultraviolet detector so as to respectively correspond to sample sizes, and calculating the content of ingredients in the rheum lhasaense sample through comparing the size of the response values with a standard sample. According to the method, the analysis and detection time of the sample can be shortened, the method has the characteristics of being good in separation effect, accurate to determine, high in sensitivity, strong in specificity, simple and convenient for an analysis method and the like, is also capable of saving the usage amount of a solvent, thus having great application prospects.
Owner:KPC PHARM INC
Method for quantitative determination of prostaglandin in biological sample
InactiveCN107607636AReduce the difficulty of separationShort analysis timeComponent separationQuantitative determinationBlood plasma
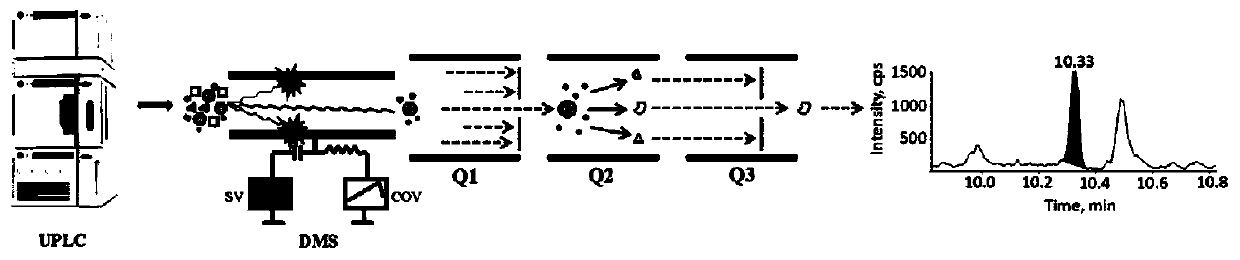
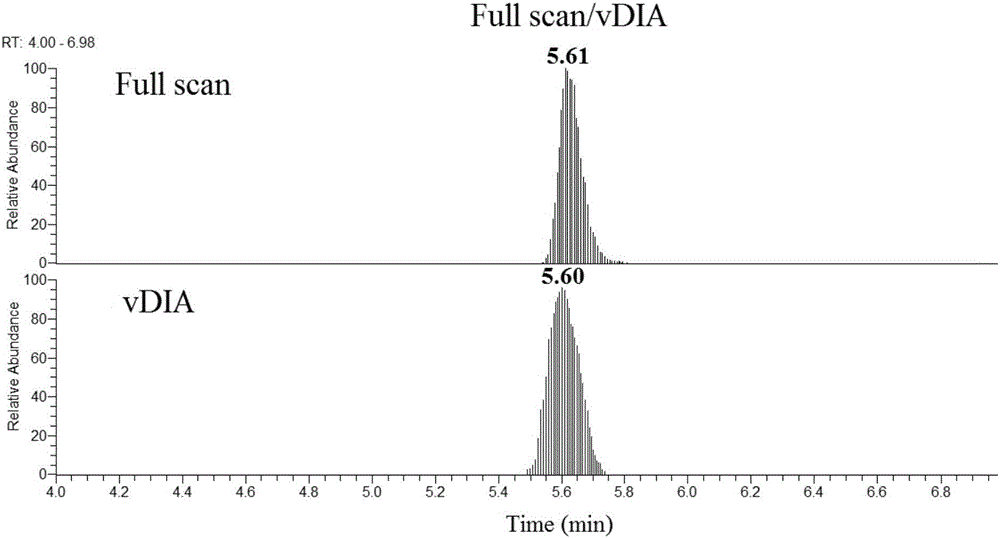
The invention discloses a method for quantitative determination of prostaglandin in a biological sample. An ultra performance liquid chromatography-differential mobility spectrometry-tandem mass spectrometry (UPLC-DMS-MS / MS) detection system is employed to determine prostaglandin in the biological sample, and a DMS pool is added between the high performance liquid chromatogram and the mass spectrum. According to the invention, the DMS pool is mounted between an ion source of the mass spectrum and a first quadrupole, the interference of limaprost in blood plasma is further removed by DMS, thusreducing the liquid chromatogram separation difficulty, changing two-dimensional chromatogram to one-dimensional chromatogram, greatly shortening the sample analysis time, and improving the sample analysis flux.
Owner:JILIN UNIV
Ultra-high performance liquid chromatography-quadrupole electrostatic field orbit ion trap mass spectrometry screening method for nutrition enhancer in milk and dairy product
Belonging to the technical field of food safety detection, the invention discloses an ultra-high performance liquid chromatography-quadrupole electrostatic field orbit ion trap mass spectrometry screening method for a nutrition enhancer in milk and dairy product. The method includes: utilizing optimized QuEChERS method to perform pretreatment on a sample; using ultra-high performance liquid chromatography-quadrupole electrostatic field orbit ion trap mass spectrometry for screening, adopting full scanning / data dependent acquisition, full scanning / variable data non-dependent acquisition, and full scanning / all-ion fragmentation acquisition scanning modes, selecting positive ion or negative ion mode scanning to obtain complete first-order and second-order spectrum; applying Exact Finder 2.5 software to process the obtained first-order and second-order spectrum, and extracting compound information; and comparing the information in the established spectrum library with the screening result of the detected object, and confirming the composition of the nutrition enhancer in the milk and dairy product. The mass spectrometer used in the invention has the main advantages of high resolution, accurate qualitative and quantitative result, high sensitivity and high quality precision, etc., and can be applied to analysis of complex matrices.
Owner:SHAANXI UNIV OF SCI & TECH
Rapid detection analysis method for statin drugs in water body
InactiveCN110133131AImprove stabilityGood repeatabilityComponent separationSolid phase extractionLipid lowering
The invention discloses a rapid detection analysis method for statin drugs in a water body, and belongs to the trace detection field of organic pollutants in the water body. In the method for detecting statin substances of lipid-lowering drugs, a solid-phase extraction small column is combined with a high performance liquid chromatography-mass spectrum method, and various statin drugs in the waterbody can be detected efficiently and quickly simultaneously. The method comprises the following steps: filtering a water sample, and adding an internal standard substance; then using the solid-phaseextraction small column to extract and purify the statin substances in the sample; and then detecting the content of a target object in the water sample through a liquid chromatogram-mass spectrometer. The method is simple in treatment steps for the water sample, convenient in operation and high in stability, and the sample applicable for the liquid chromatograph / mass spectrometer can be obtainedquickly; the pretreatment cost of the sample is low, rapid and efficient detection can be carried out, and the method is high in detection speed, high in degree of automation, sensitive in response and convenient for industrial application, is a simple, convenient, rapid and accurate qualitative and quantitative detection method, and is applicable for popularization and application.
Owner:SHANGHAI UNIV
HPLC (high performance liquid chromatography) method for detecting concentration of voriconazole in human plasma
PendingCN109406644AMeet the determination requirementsSimple and fast operationComponent separationHplc methodBlood plasma
The invention discloses an HPLC (high performance liquid chromatography) method for detecting concentration of voriconazole in human plasma and relates to the technical field of medical treatment. TheHPLC method is established for detecting the concentration of voriconazole in the human plasma, which aims to provide reference for clinical rational drug use. The method comprises steps as follows:determination of chromatographic conditions, preparation of solutions, treatment of plasma samples, retention of voriconazole and internal standard chlorzoxazone, taking of an appropriate quantity ofa voriconazole stock solution, preparation of voriconazole, preparation of voriconazole, and taking of quality control plasma samples with low, medium and high mass concentrations. The method is simple to operate, has an accurate result, is suitable for detecting voriconazole in human plasma, and can assist in avoiding poor curative effect or increased adverse reactions caused by too low or too high plasma concentration. An experimental result of the HPLC method for detecting the concentration of voriconazole in the human plasma meets the measuring requirement of biological samples and is suitable for concentration monitoring of clinical treatment and pharmacokinetic study.
Owner:史长城
Method for detecting 2,2,6,6-tetramethylpiperidinooxy with high performance liquid chromatography-mass spectrometry
InactiveCN109632981AEasy to operateThe test result is accurateComponent separationConcentration gradientLinear regression
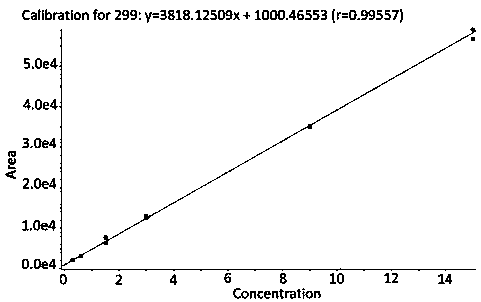
The invention discloses a method for detecting 2,2,6,6-tetramethylpiperidinooxy with high performance liquid chromatography-mass spectrometry. The method comprises the steps that 1, a test solution and a reference stock solution is prepared; 2, the test solution and the reference stock solution with a certain concentration gradient are subjected to sample introduction, a high performance liquid chromatograph mass spectrometer is used for detecting and recording a chromatogram; and 3, linear regression analysis is performed on the mass concentration of the reference stock solution and chromatogram peak area, a regression equation and correlation coefficients are obtained, and a standard curve is manufactured; and the peak area of 2,2,6,6-tetramethylpiperidinooxy in the chromatogram of the test solution is utilized to calculate the content of 2,2,6,6-tetramethylpiperidinooxy by an external standard method. The method provided by the invention is the first method for detecting 2,2,6,6-tetramethylpiperidinooxy developed in the field. The method has accurate detection result, high sensitivity, good stability, and low detection limit, and totally meets detection requirements for genotoxic impurities in the field.
Owner:CHANGZHOU HEQUAN PHARMA CO LTD
High performance liquid chromatography analyzing method of eplerenone related substances
The invention discloses a high performance liquid chromatography analyzing method of eplerenone related substances. The high performance liquid chromatography analyzing method is characterized in that a reversed-phase chromatographic column and an ultraviolet detector are used and an acetonitrile-methanol-phosphate buffer solution is used as the flowing phase to perform isocratic elution. The high performance liquid chromatography analyzing method has the advantages that all the known impurities in eplerenone raw materials and eplerenone preparations can be analyzed at the same time, the content of the known impurities can be effectively controlled through the self-contrasted method of the main component added with correction factors, the separation degree between a main peak and an impurity peak adjacent to the main peak and between every two impurity peaks is larger than 1.5, and the purity of the main peak and the impurity peaks is 1.0. By the high performance liquid chromatography analyzing method, a simple and reliable analyzing method is provided for the quality control analyzing of the eplerenone raw materials and the eplerenone preparations.
Owner:HEFEI JIUNUO MEDICAL TECH
High-performance liquid chromatography-mass spectroscopy of iprodione in tobacco and tobacco products
The invention discloses a high-performance liquid chromatography-mass spectroscopy of iprodione in tobacco and tobacco products. The high-performance liquid chromatography-mass spectroscopy is characterized by comprising the following steps: completely decomposing iprodione in a sample as metabolite [iprodione-des-(N-isopropyl formamide)], acidizing the metabolite by using hydrochloric acid, performing liquid-liquid extraction by using dichloromethane, filtering, nitrogen-blowing to proximate dry, re-dissolving by using acetonitrile, measuring the iprodione metabolite content in the sample byusing chromatography-mass spectroscopy, thereby directly measuring the content of iprodione in the sample. Through the method disclosed by the invention, the disadvantage of a sample treating method in the prior art is overcome, the blank of the substance measurement is filled, thereby providing various references for the measurement of related residual limit and the development of the method technology; the sample pretreatment method and an instrument detection method are optimized for the tobacco sample, and the method disclosed by the invention has the following effects in comparison with the prior art: the sample pretreatment process of the method is simple and fast, and the method has the advantages of being accurate in operation, high in sensitivity and good in repeatability.
Owner:CHINA NAT TOBACCO QUALITY SUPERVISION & TEST CENT
Method for detecting content of oxaloacetic acid on basis of high performance liquid chromatography
InactiveCN108645936AHigh precisionHigh detection sensitivityComponent separationUltraviolet detectorsHigh-performance Liquid Chromatography-UV
The invention discloses a method for detecting the content of oxaloacetic acid on the basis of high performance liquid chromatography. According to a principle,after oxaloacetic acid reacts with a derivative agent to generate an ultraviolet absorbing substance,the ultraviolet absorbing substance is separated by means of the high performance liquid chromatography,and the content of the ultravioletabsorbing substance is detected through an ultraviolet detector. A high performance liquid chromatographic instrument needed to be used in the method has high precision,can detect the nanogram-scale content and has high detection sensitivity. A sample pretreatment process is simple and easy to operate. An extract has simple compositions,only a water solution is needed for extraction,and the cost is low. Detecting steps of the method are automatically operated through the instrument,hundreds of samples can be detected continuously,accurate data is read,and the time for detecting each sample isshort. Compared with a biological immunization detection method,the method has the advantages that the price advantage is obvious,and required reagents are basically free of toxicity.
Owner:SUZHOU COMIN BIOTECH
High performance liquid chromatography-based method used for detection of eugenol in cigarette main stream smoke
ActiveCN103336085AAvoid interferenceFast wayComponent separationCorrelation coefficientLinear correlation
The invention discloses a high performance liquid chromatography-based method used for detection of eugenol in cigarette main stream smoke. The method comprises steps such as preparation of standard working solutions and sample solutions, high performance liquid chromatography analysis, and calculation of analysis results. External standard method is used for quantification in the method; the optimized detection method is simple and accurate; the chromatographic peak of the target component and the chromatographic peaks of other components can be separated easily because of adopted chromatographic conditions; when the concentration range is 0.055 to 5.5 mg / l, linear dependence of the standard curve is relatively good, correlation coefficient R2 is 0.9996, detection limit is 0.015 ug per cigarette, average relative standard deviation is 4.69%, and adding standard recovery is 82.67 to 97.46%. It can be concluded from the characteristics above that: sensitivity, repeatability and recovery rate of the method are high, and the method is suitable for qualitative and quantitative analysis of eugenol in cigarette main stream smoke.
Owner:CHINA TOBACCO JIANGSU INDAL
Method for detecting content of sulphobetaine surfactant in oil well injection-production fluid through high performance liquid chromatography
InactiveCN108593798AAccurate measurementHigh sensitivityComponent separationInterference factorGradient elution
The invention relates to a method for detecting the content of a sulphobetaine surfactant in an oil well injection-production fluid through high performance liquid chromatography. The method comprisespreparing a standard substance solution and a sample solution from a blank oil well injection-production fluid, feeding 20 micro-liter of the sample, and calculating the content through an external standard method, wherein the high performance liquid chromatography conditions comprise a C18 chromatographic column as a chromatographic column, an evaporative light scattering detector as a detector,methanol and water as mobile phases, use of gradient elution with 70% methanol in 0-2min and 100% methanol after 2.01min, airflow velocity of 2-2.5L / min and an evaporating temperature of 110-120 DEGC. The method has the advantages of fast analysis speed, simple operation, safety, reliability, quantification of the content of a sulphobetaine surfactant based on an external standard method, few interference factors, high accuracy, good repeatability and high sensitivity.
Owner:LANZHOU INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method for measuring Farnesal contents in medicine loading micelle by high performance liquid chromatography
InactiveCN109884216ATest accurateSimple and fast operationComponent separationColumn temperatureWavelength
The invention relates to a method for measuring Farnesal contents in medicine loading micelle by high performance liquid chromatography, and belongs to the field of medicine analysis. An Agilent-1260high performance liquid chromatograph and an ultraviolet detector are adopted, a detection wavelength is 216nm, and a chromatographic condition is that a chromatographic column is a reverse chromatographic column which selects octadecylsilane chemically bonded silica as a filler; a moving phase is preferably mixture of acetonitrile and water, wherein the volume ratio of acetonitrile to water is 80:20; and a flow rate is 0.8-1.2mL / min, a sample introduction amount is 5-15muL, and a column temperature is 20-40DEG C. The method has the characteristics of being simple in operation, high in specificity, high in accuracy, good in precision and the like, and an intra-day and daytime standard deviation does not exceed 1.2%. Therefore, by use of the method, the requirement that Farnesal contents can be accurately and effectively measured can be met, and the method can be accurately used for testing Farnesal contents in experiments, including the encapsulation efficiency, the medicine loading capacity, the in vitro release and the like of the Farnesal-loaded medicine loading micelle.
Owner:SOUTHWEST MEDICAL UNIVERISTY
Method and application of detecting sulfonamide antibiotic by adopting high performance liquid chromatography
InactiveCN108414663AAnalysis method is simpleAnalytical method is fastComponent separationAfter treatmentSorbent
The invention belongs to the technical field of chemical substance residue detection and particularly relates to a method and application of detecting a sulfonamide antibiotic by adopting high performance liquid chromatography. By taking a high performance liquid chromatograph as a platform, the invention provides concrete chromatographic condition and sample pretreatment, qualitative and quantitative analysis methods of several sulfonamide antibiotics in a water body before and after treatment with an adsorbent. The analysis method provided by the invention is simple, convenient and rapid, high in sensitivity, and good in selectivity and repeatability and can be suitable for any water body treated with the adsorbent.
Owner:SOUTH CHINA NORMAL UNIVERSITY +1
Method for measuring multiple preservatives in food by adopting high performance liquid chromatography
The invention discloses a method for measuring multiple preservatives in a food by adopting high performance liquid chromatography. The method comprises the following steps of pretreating a sample: dissolving the to-be-measured sample in an organic solvent according to a certain mass ratio, and taking supernate to perform filtering after centrifugation, wherein obtained filtrate is a to-be-measured solution; preparing experimental conditions: selecting a chromatographic column, performing startup preheating, and preparing a solution used as a mobile phase and a standard solution; and measuringand analyzing the preservatives: injecting a certain volume of the standard solution and the to-be-measured solution into a high performance liquid chromatograph, controlling a flow speed, obtaininga to-be-measured curve and a standard curve, and analyzing the content of the preservatives in the to-be-measured curve according to the standard curve. According to the method, by selecting a reasonable reagent as the mobile phase and a proper method, the sample is treated, and the preservatives in the food are effectively purified, so that the influence of the reagent on a detection result is avoided, and the accuracy of a final result is ensured.
Owner:苏州汉德瑞生物工程有限公司
High-performance liquid chromatography method for measuring nucleotide in milk powder
ActiveCN102680601BThe separation method is simpleEasy to separateComponent separationFood additiveHydrogen Nitrate
Owner:SHANDONG KAISHENG NEW MATERIALS
Method adopting ultra-high performance liquid chromatography-quadrupole static electric field track ion trap mass spectrum for screening corrosion remover in milk and dairy products
InactiveCN106290694AEasy to operateFast positive and negative switchingComponent separationQuadrupoleSpectrometer
The invention discloses a method adopting an ultra-high performance liquid chromatography-quadrupole static electric field track ion trap mass spectrum for screening a corrosion remover in milk and dairy products, and belongs to the technical field of food safety testing. An optimized QuEChERS method is adopted for preprocessing a sample; the ultra-high performance liquid chromatography-quadrupole static electric field track ion trap mass spectrum is applied for performing screening, full-scanning / data dependency collection, full-scanning / variable data independent collection and a full-scanning / full-ion fragmentation collection scanning mode are adopted, positive ion or negative ion mode scanning is selected, and complete first-order or second-order spectra are obtained; Exact Finder 2.5 software is applied for processing the obtained first-order or second-order spectra, and compound information is extracted. Information in a building spectrum library and a tested substance screening result are compared, and the composition of the corrosion remover in milk and dairy products is ensured. An adopted mass spectrometer has the main advantages of being high in resolution, accurate in qualitative and quantitative result, high in sensitivity, high in quality precision and the like and can be applied to complex matrix analysis.
Owner:SHAANXI UNIV OF SCI & TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com