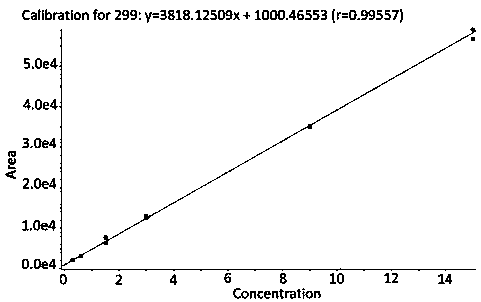
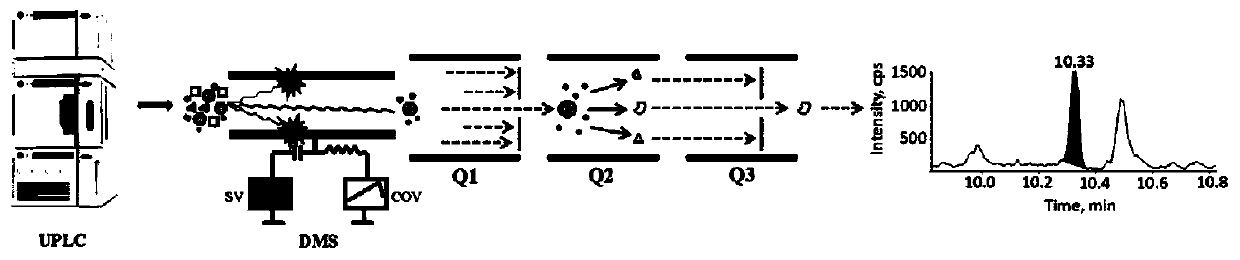
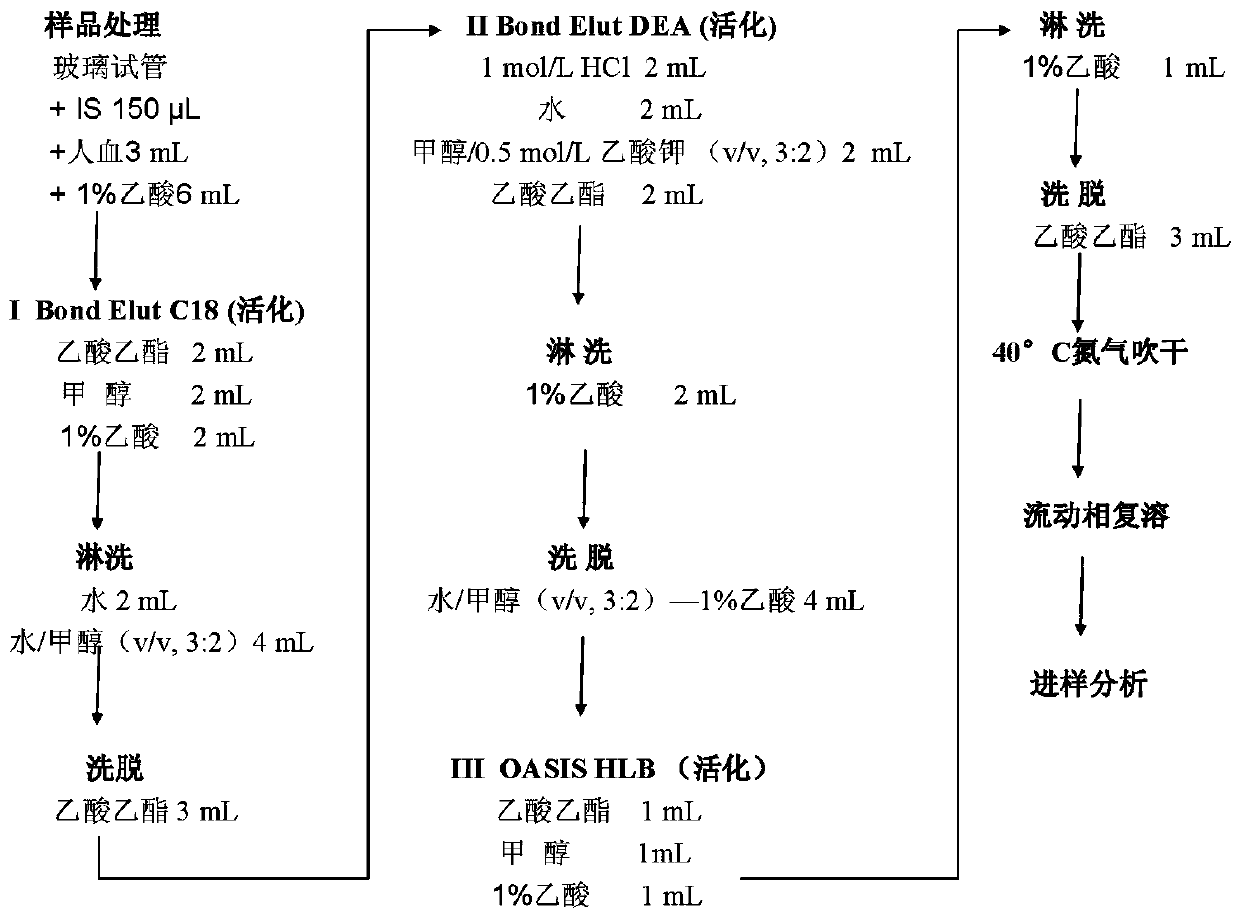
Method for quantitative determination of prostaglandin in biological sample
A biological sample and prostaglandin technology, which is applied in the field of drug analysis and research, can solve problems such as unsatisfactory analysis requirements and unreachable sensitivity of injection volume, and achieve the effects of shortening sample analysis time, increasing throughput, and reducing separation difficulty
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] In this example, Limaprostaglandin in plasma was specifically determined.
[0060] Healthy volunteers took 5 μg of test preparation limaprostaglandin tablets orally on an empty stomach, before administration 0h (within 30 minutes before administration) and after administration 10min, 15min, 20min, 25min, 30min, 40min, 1h, 1.5h, Venous blood was collected at 13 time points of 2h, 3h, 4h, and 6h. Each time, 14mL of blood (2 tubes in total) was collected, and slowly placed in an anti-adsorption test tube treated with sodium heparin, and centrifuged at 1800g for 5min in a 4°C centrifuge. . After centrifugation, the plasma was transferred to anti-adsorption cryovials. Determination of the content of limaprostaglandin in plasma, plasma drug concentration-time curve see Figure 4 .
[0061] The main steps are as follows:
[0062] A. Treatment of biological samples to be tested:
[0063] Take 3 mL of plasma sample, put it in a 10 mL anti-adsorption glass tube, add 100 μL o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| collision energy | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com