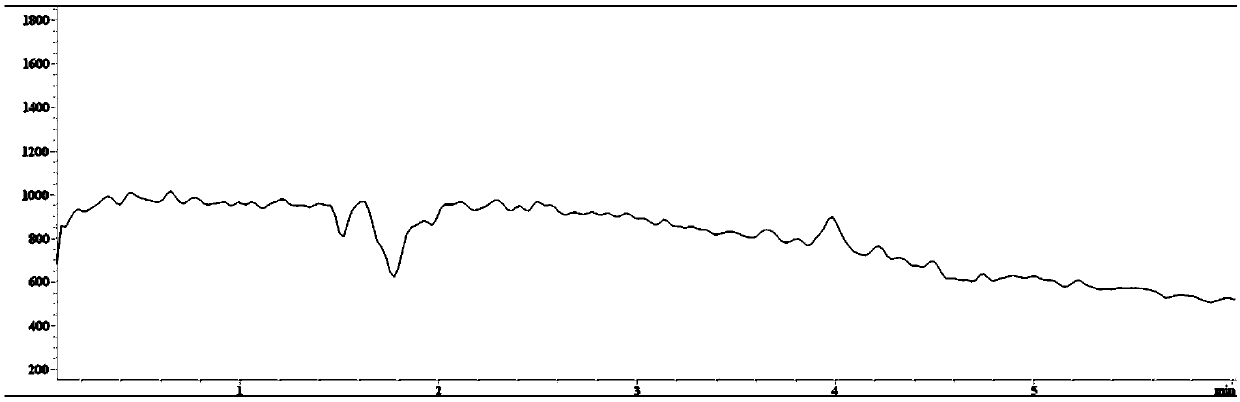
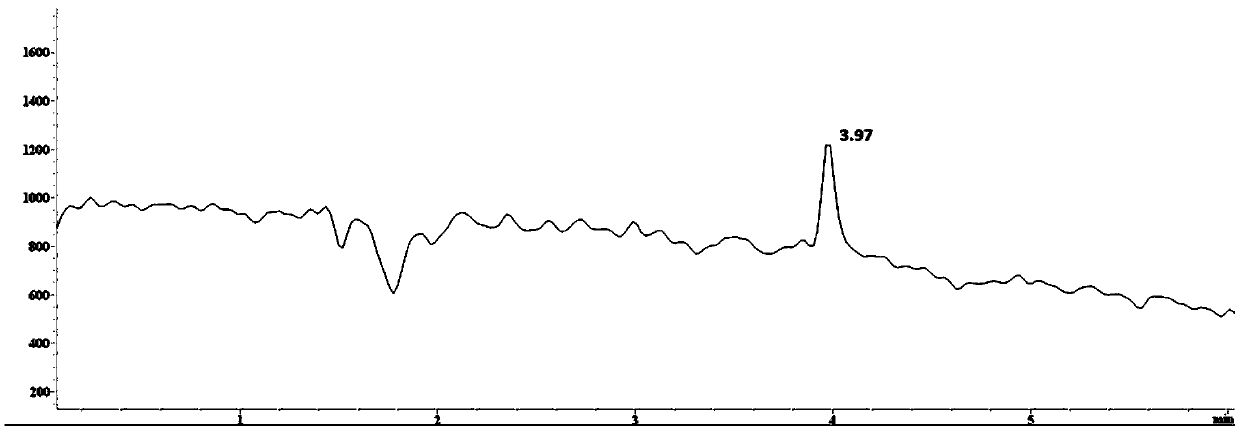
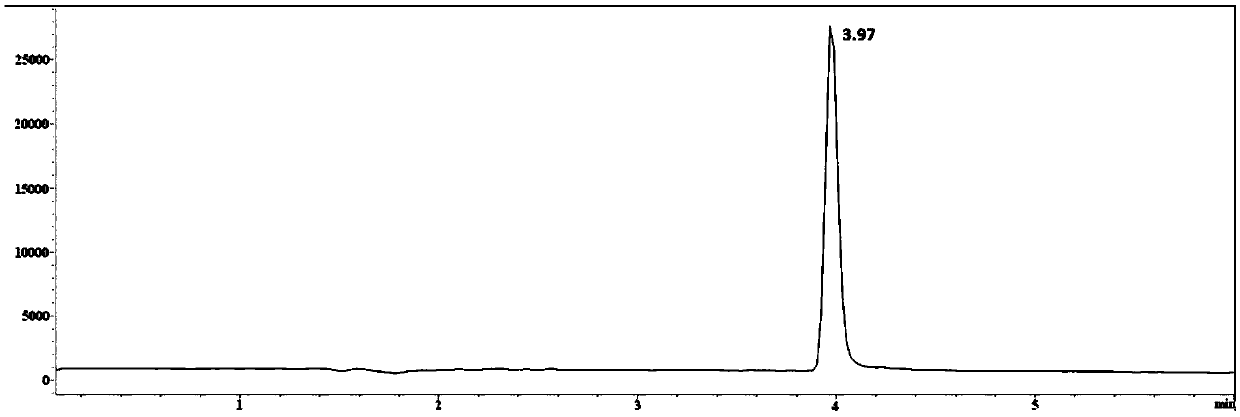
Method for detecting 2,2,6,6-tetramethylpiperidinooxy with high performance liquid chromatography-mass spectrometry
A technology of tetramethylpiperidine and nitrogen oxides, which is applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems of weak ultraviolet absorption, low sensitivity, and inaccurate detection results, and achieve accurate detection results, high sensitivity, Simple operation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] 1. Instruments and Reagents
[0031] instrument:
[0032] HPLC-MS: Agilent 6525B HPLC-Quadrupole-TOF Mass Spectrometer Parts per million Analytical Balance: Mettler Toledo XSE205DU
[0033] Reagent:
[0034] Water: Millipore ultrapure water
[0035] Acetonitrile: Merck chromatography pure
[0036] Formic acid: J&K chromatographically pure
[0037] Sodium ascorbate: Damas-beta analytically pure
[0038] 2,2,6,6-tetramethylpiperidine nitrogen oxide: Aldrich, content 98%
[0039] 2. Detection conditions of high performance liquid chromatography-mass spectrometry
[0040] High performance liquid chromatography conditions:
[0041] Chromatographic column: Waters Xbridge Phenyl (150×4.6mm, 3.5μm);
[0042] Mobile phase A: aqueous solution containing 0.1% formic acid by volume fraction;
[0043] Mobile phase B: acetonitrile solution containing 0.1% formic acid by volume fraction;
[0044] Flow rate: 1.0mL / min;
[0045] Column temperature: 40°C;
[0046] Injection v...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com