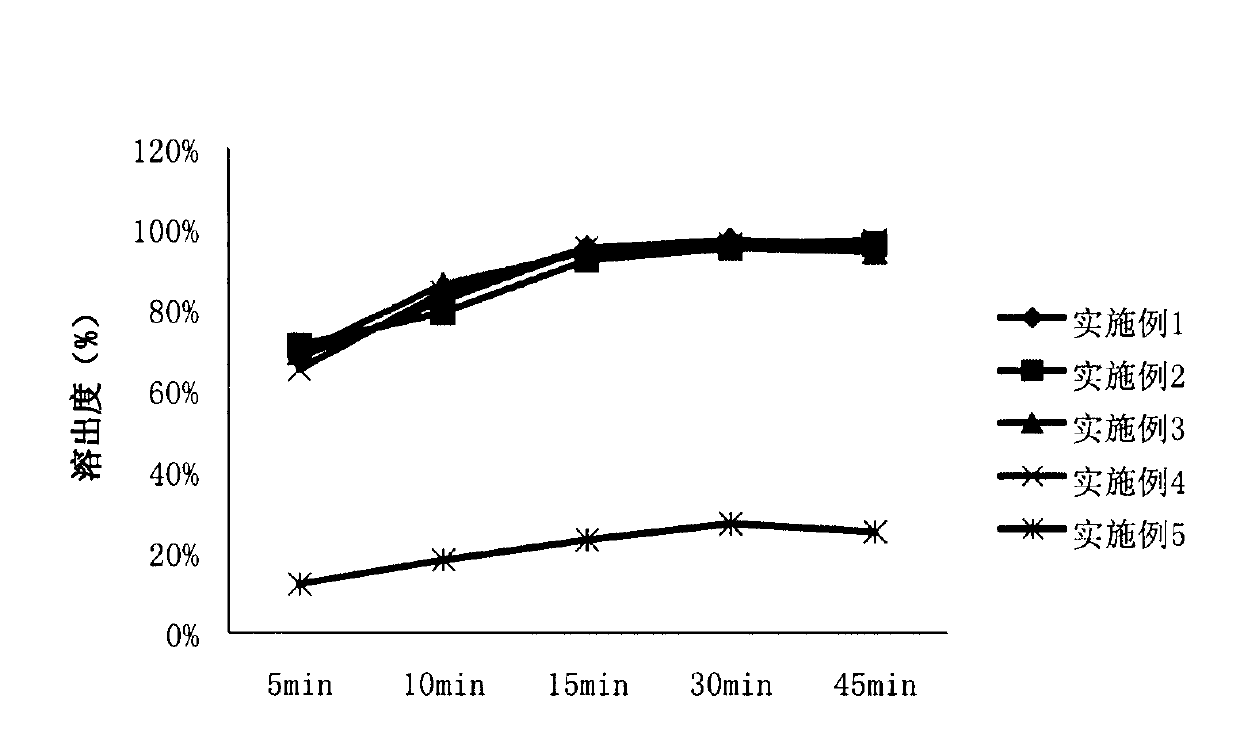
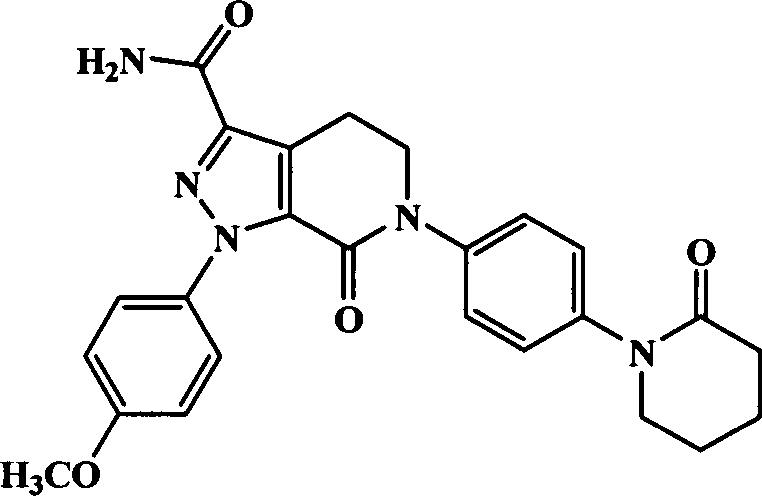
Apixaban tablet
A technology of apixaban tablet and water-soluble carrier, which is applied in the field of medicine, can solve the problems of drug absorption influence, low in vitro dissolution rate, low bioavailability, etc., and achieves convenience for large-scale production, good reproducibility, and increased drug absorption effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] This example provides the prescription and preparation method for preparing 10,000 Apixaban tablets (specification: 2.5 mg / tablet) as follows
[0022] Preparation:
[0023] (1) Take polyethylene glycol 6000 of recipe quantity, heat to melt at 60 ℃;
[0024] (2) Add the apixaban of the prescribed amount into the melt obtained in step (1) under slow stirring conditions, stir vigorously after adding all of it, the slow stirring is 200~400r / min, and the vigorous stirring is 10000~20000r / min;
[0025] (3) rapidly cooling and solidifying the melt obtained in step (2) for 2 hours at -20°C;
[0026] (4) Grinding the cured product obtained in step (3) at 0°C and passing through an 80-mesh sieve; drying the crushed product at 20°C for 4 hours under reduced pressure to obtain apixaban solid dispersion;
[0027] (5) Mix the apixaban solid dispersion obtained in the step (4) with the prescribed amount of microcrystalline cellulose; crospovidone; and micropowdered silica gel eve...
Embodiment 2
[0029] This example provides the prescription and preparation method for preparing 10,000 Apixaban tablets (specification: 2.5 mg / tablet) as follows
[0030] Preparation:
[0031] (1) Take polyethylene glycol 6000 of recipe quantity, heat to melt at 60 ℃;
[0032] (2) Add the apixaban of the prescribed amount into the melt obtained in step (1) under slow stirring conditions, stir vigorously after adding all of it, the slow stirring is 200~400r / min, and the vigorous stirring is 10000~20000r / min;
[0033] (3) rapidly cooling and solidifying the melt obtained in step (2) for 2 hours at -20°C;
[0034] (4) Grinding the cured product obtained in step (3) at 0°C and passing through an 80-mesh sieve; drying the crushed product at 20°C for 4 hours under reduced pressure to obtain apixaban solid dispersion;
[0035] (5) Mix the apixaban solid dispersion obtained in the step (4) with the prescribed amount of microcrystalline cellulose; crospovidone; and micropowdered silica gel eve...
Embodiment 3
[0037] This example provides the prescription and preparation method for preparing 10,000 Apixaban tablets (specification: 2.5 mg / tablet) as follows
[0038] Preparation:
[0039] (1) Take polyethylene glycol 6000 of recipe quantity, heat to melt at 60 ℃;
[0040] (2) Add the apixaban of the prescribed amount into the melt obtained in step (1) under slow stirring conditions, stir vigorously after adding all of it, the slow stirring is 200~400r / min, and the vigorous stirring is 10000~20000r / min;
[0041] (3) rapidly cooling and solidifying the melt obtained in step (2) for 2 hours at -20°C;
[0042] (4) Grinding the cured product obtained in step (3) at 0°C and passing through an 80-mesh sieve; drying the crushed product at 20°C for 4 hours under reduced pressure to obtain apixaban solid dispersion;
[0043] (5) Mix the apixaban solid dispersion obtained in the step (4) with the prescribed amount of microcrystalline cellulose; crospovidone; and micropowdered silica gel eve...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com