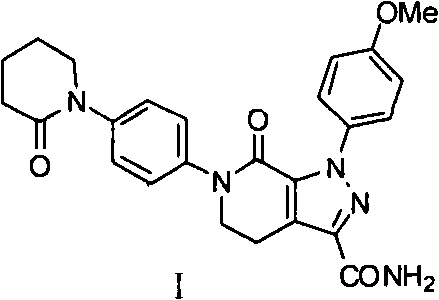
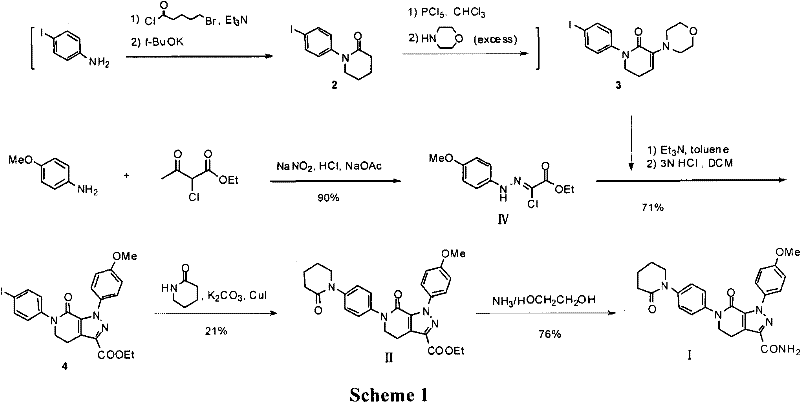
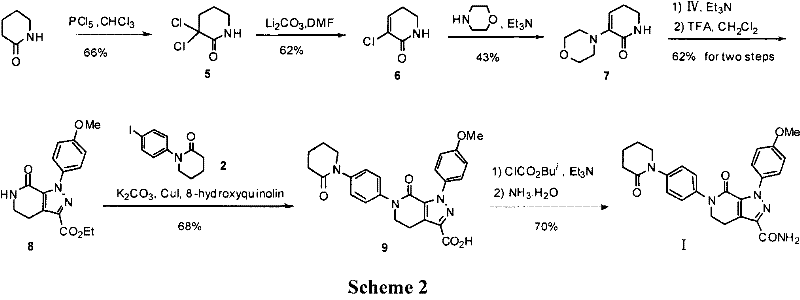
Method for preparing antithrombotic medicament apixaban
An antithrombotic drug, the technology of Apixaban, which is applied in the field of preparation of antithrombotic drug Apixaban, can solve the problems of unfavorable and economical preparation, large amount of auxiliary reagents, and difficult availability of intermediates, so as to avoid expensive raw materials and auxiliary materials. Use of reagents, shortening of reaction time, and improvement of reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] (1) Preparation of Compound V
[0075]
[0076] 40ml of anhydrous tetrahydrofuran was added into the reaction flask, and p-nitroaniline (11.04g, 0.08mol) and triethylamine (22ml, 0.16mol) were sequentially added under stirring. The reaction solution was cooled to 0°C with an ice-salt bath, and a mixed solution composed of 5-chlorovaleryl chloride (16ml, 18.6g, 0.12mol) and 20ml of anhydrous tetrahydrofuran was slowly added dropwise, and the temperature of the system was controlled at 0 to 5 °C range. After the addition, the reaction solution was slowly warmed up to room temperature for 4 hours, followed by TLC until the p-nitroaniline spots disappeared. The reaction solution was cooled to 0°C with an ice-salt bath, and NaH solid (5.76 g, 0.24 mol) was slowly added within 10 minutes. After the addition, the reaction solution was slowly warmed up to room temperature for 1 hour, followed by TLC until the intermediate spots disappeared. Stop the reaction, remove the so...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com