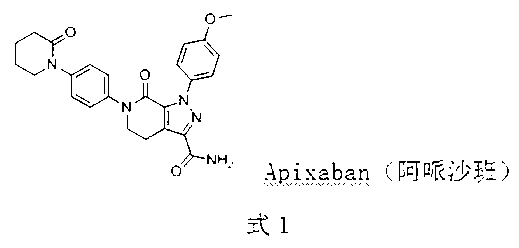
Preparation method of Apixaban as anti-thrombotic drug
The technology of an antithrombotic drug, apixaban, is applied in the field of preparation of organic compounds, can solve the problems of large amount of auxiliary reagents, complicated routes, difficult to obtain intermediates and the like, and achieves short steps, improved reaction yield and mild reaction conditions. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
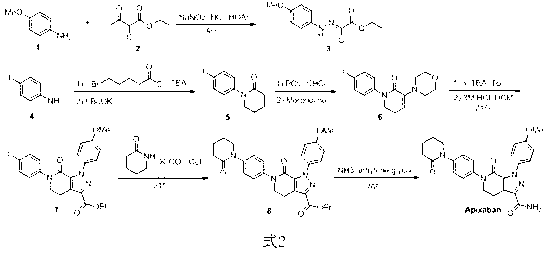
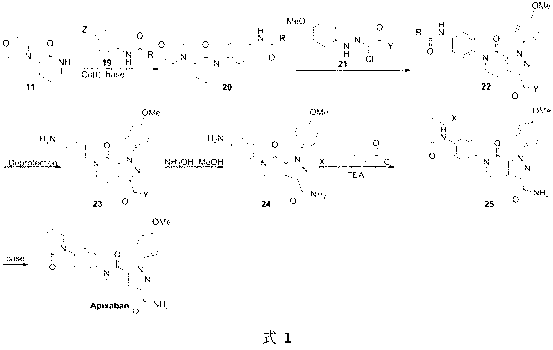
[0026] Example 1: In the synthetic route shown in formula 7, R is tert-butoxy, and removing the amino protecting group is trifluoroacetic acid, dichloromethane solution, X is a bromine atom, Y is an ethoxy group, and Z is an iodine atom; compound 11 converted to compound 20a The inorganic base used is cesium carbonate; the compound
[0027]
[0028] 25a converted to compound Apixaban The base used was sodium hydrogen.
[0029] Preparation of compound 20a
[0030] In a 10mL single-necked bottle, the compound 11(0.182g), compound 19a (0.383g) was dissolved in 4.6mL of dioxane, cesium carbonate (0.65g) and catalytic amount of N,N'-dimethylethylenediamine (0.018g) were added successively. A catalytic amount of cuprous iodide (0.019 g) was added under argon protection. The reaction mixture was heated to 110 °C for 11 hours, cooled to room temperature, 40 mL of water was added to the reaction solution, extracted with ethyl acetate (20 mL×2), washed with saturated brine...
Embodiment 2
[0038] Embodiment 2: In the synthetic route shown in formula 8, R is a trifluoromethyl group, and the amino protecting group is removed with a methanol solution of potassium carbonate, X is a chlorine atom, Y is a tert-butoxy group, Z is a bromine atom,
[0039]
[0040] compound 11 convert to compound 20b The inorganic base used is potassium phosphate; compound 25b convert to compound Apixaban The base used was sodium hydroxide.
[0041] Preparation of compound 20b
[0042] Compounds in 10mL single-neck vials 11 (0.182g), compound 19b (0.378g) was dissolved in 4.6mL of dioxane, followed by adding anhydrous potassium phosphate (0.42g) and catalytic amount of N,N'-dimethylethylenediamine (0.018g). A catalytic amount of cuprous iodide (0.019 g) was added under argon protection. The reaction mixture was heated to 110 °C for 11 hours, cooled to room temperature, 40 mL of water was added to the reaction solution, extracted with ethyl acetate (20 mL×2), washed with sat...
Embodiment 3
[0052] Embodiment three: In the synthetic route shown in formula 9, R is benzyloxy, and the amino protecting group is removed with hydrochloric acid solution, X is iodine atom, Y is isopropoxy group, Z is iodine atom, compound 11
[0053] convert to compound 20c The inorganic base used is sodium carbonate; compound 25c convert to compound Apixaban The base used was potassium tert-butoxide.
[0054] Preparation of compound 20c
[0055] Compounds in 10mL single-neck vials 11 (0.182g), compound 19c (0.378g) was dissolved in 4.6mL of dioxane, and anhydrous sodium carbonate (0.40g) and a catalytic amount of N,N'-dimethylethylenediamine (0.018g) were added successively. A catalytic amount of cuprous iodide (0.019 g) was added under argon protection. The reaction mixture was heated to 110 °C for 11 hours, cooled to room temperature, 40 mL of water was added to the reaction solution, extracted with ethyl acetate (20 mL×2), washed with saturated brine, combined with the orga...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com