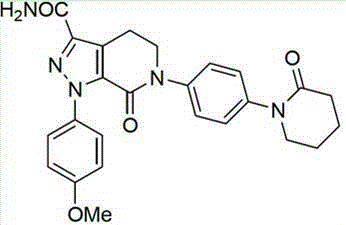
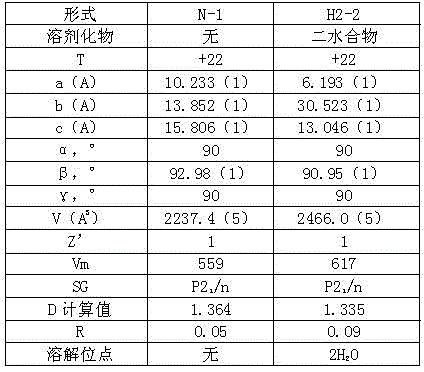
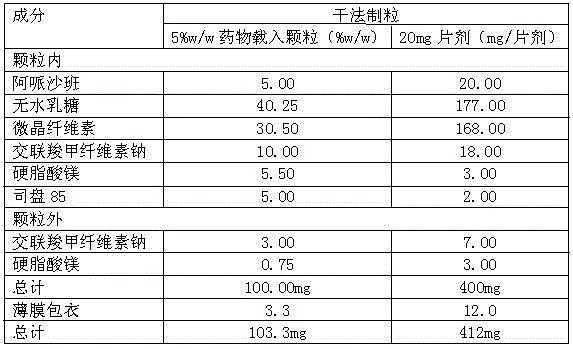
Apixaban composition and preparation method thereof
A technology of apixaban and apixaban tablets, applied in the field of apixaban pharmaceutical preparations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0031] The dry granulation process for preparing crystalline apixaban granules having an average particle size equal to or less than about 70 μm is believed to be novel and is therefore provided as a further feature of the present invention. Therefore, the present invention provides a pharmaceutical product preparation method, comprising the following steps:
[0032] (1) blending the raw materials prior to granulation;
[0033] (2) Granulate the raw materials of step 1 using dry or wet granulation methods;
[0034] (3) blending the granules of step 2 with extragranular raw material;
[0035] (4) compressing the blend of step 3 into tablets; and
[0036] (5) Film-coat the tablets in step 4.
[0037] In another embodiment, the present invention provides a method for preparing a pharmaceutical product, comprising the steps of:
[0038] (1) blending the raw material with apixaban of controlled particle size;
[0039] (2) Including intragranular portions of binder, disi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Average particle size | aaaaa | aaaaa |
| D90 | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com