Synthetic method for Apixaban drug intermediate
A synthetic method, the technology of apixaban, applied in the field of synthesis, to achieve the effects of reducing the generation of impurities, avoiding reaction conditions, and improving reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
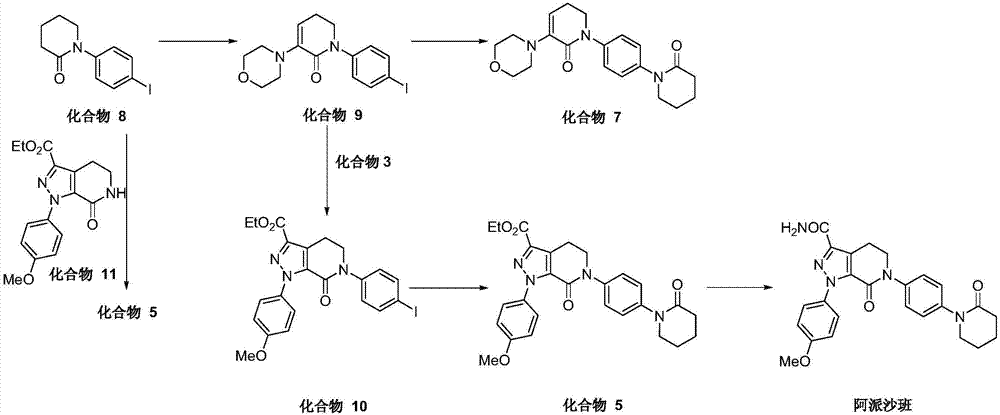
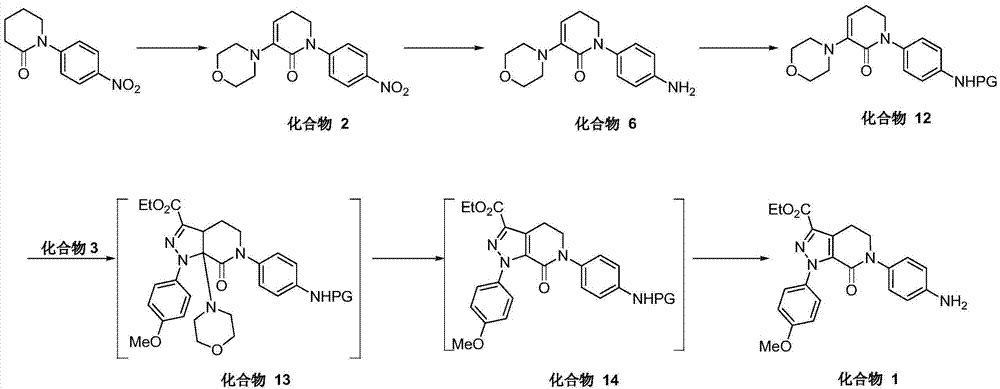
Image
Examples
Embodiment 1
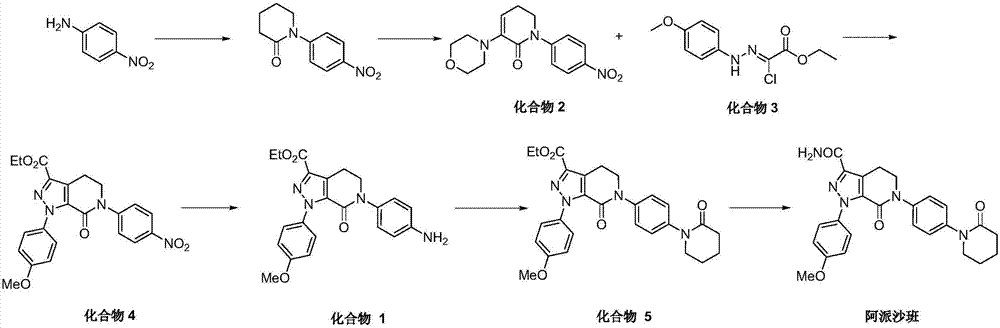
[0057] Synthesis of 1-(4-nitrophenyl) piperidone:
[0058] P-nitroaniline (100g, 0.724mol) was added to tetrahydrofuran (500mL), triethylamine (146.5g, 1.448mol) was added, and the reaction flask was replaced with nitrogen for protection. Begin to cool down to the internal temperature of -5-0°C, add 5-chlorovaleryl chloride (140.3g, 0.905mol) in tetrahydrofuran (180mL) solution dropwise to the reaction solution, control the temperature at -5-5°C, add dropwise to 0- Incubate at 5°C for half an hour, then heat to 23-25°C, and stir for 2 hours. The internal temperature was lowered to -5-0°C, solid potassium tert-butoxide (223.4g, 1.991mol) was added in batches, the temperature was controlled at 0-25°C, the temperature was raised to 23-25°C after the addition, and the stirring was continued for 12 hours. The reaction solution was concentrated to dryness, 600 mL of dichloromethane was added, 800 mL of water was added, and the separation was carried out. The aqueous phase was extracte...
Embodiment 2
[0061] Synthesis of compound 2:
[0062] The dichloromethane solution obtained in Example 1 was added to the reaction flask, the internal temperature was controlled at 20-25° C., and solid phosphorus pentachloride (560 g, 2.69 mol) was added in batches. The reaction was violent, smoking and heating up. After the addition was completed, the reaction was heated to reflux, and the stirring was continued for 1 hour. The reaction solution was cooled to 20-25°C, and the reaction solution was slowly poured into stirring ice water (1.2L) for extraction. The layers were separated, the aqueous phase was extracted with 400 mL of dichloromethane, and the organic phases were combined. Wash with saturated sodium bicarbonate to pH 7-8, then wash with 500 mL of brine. The organic phase was concentrated to viscous, and 440 mL of methyl tert-butyl ether was added under mechanical stirring. A large amount of solids precipitated, filtered with suction, the filter cake was washed with (100 mL) met...
Embodiment 3
[0067] Synthesis of compound 6:
[0068] Sodium sulfide nonahydrate (76g, 0.32mol) was added to 500mL water, heated to 60-65°C and stirred to dissolve. Then the compound 2 (100 g, 0.33 mol) obtained in Example 2 was added. After the addition, the internal temperature of the reaction was raised to 75-80° C. and the reaction was stirred for 2 hours. Add sodium sulfide nonahydrate (40g, 0.167mol) and continue to stir for 2 hours. A total of 4 supplements of sodium sulfide nonahydrate, 40g each time, continue stirring for 2 hours after each supplement. After all four additions were completed, the reaction was continued with stirring at 75-80°C for 12 hours. Cool down to 45℃-50℃ and filter with suction. The filter cake was slurried with 500 mL of water for 1 hour, and then filtered with suction. The filter cake was slurried with 400 mL of ethanol for 1 hour, and then filtered with suction. The filter cake was vacuum dried at 50°C for 12 hours to obtain 83.9 g of compound 6 with a ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com