Method for preparing apixaban
A technology of apixaban and a refining method, which is applied in the field of preparation of apixaban, can solve the problems of low yield and low purity, achieve the effects of less by-products, simple and convenient operation methods, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
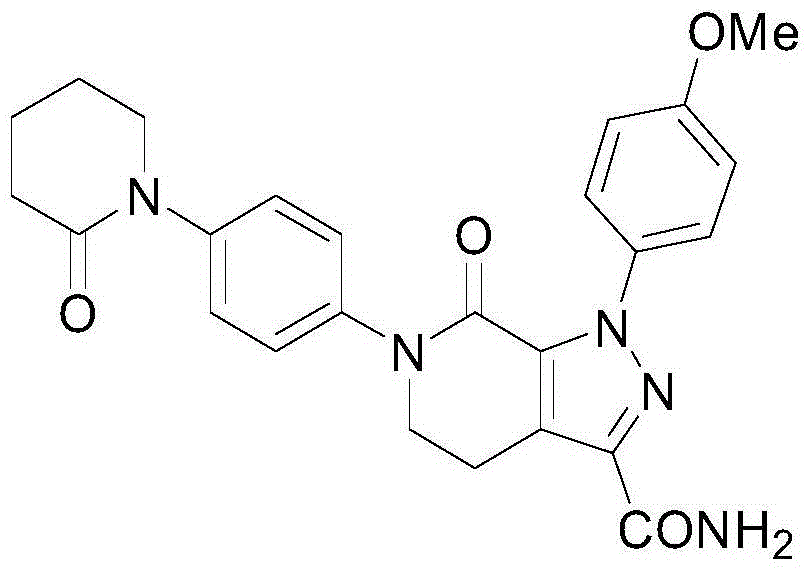
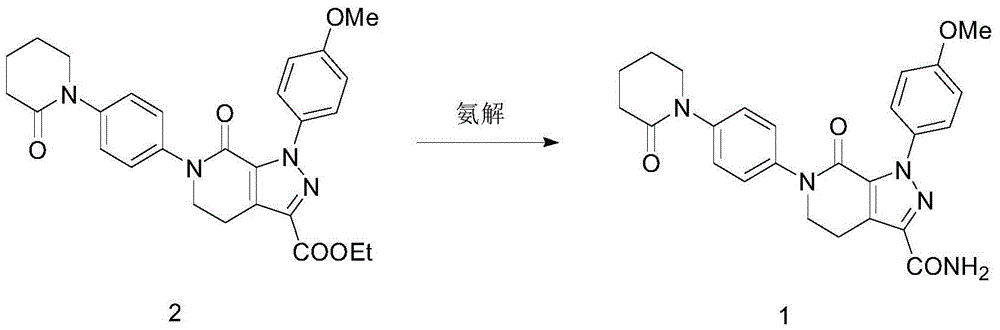
[0030] Compound (2) (4.9g) and 150mL of ethanol were sequentially added into a stainless steel autoclave, sealed, started to pass ammonia gas, heated to 60°C, kept the pressure at 0.3MPa, reacted for 8 hours, and stopped the reaction. After the reactor was cooled to room temperature, it was opened, filtered, and the filter cake was washed with ethanol. After drying, 4.32 g of white solid mp236-239°C was obtained. The yield was 94.02%, and the HPLC purity was 98.31%.
[0031] Ethanol refining: volume of ethanol: 1-(4-methoxyphenyl)-7-oxo-6-[4-(2-oxo-1-piperidinyl)phenyl]-4,5,6, 7-Tetrahydro-1H-pyrazolo[3,4-C]pyridine-3-carboxamide Mass=30ML: 1G, stir and heat to reflux, the system is clear, filter the insoluble matter while it is hot, and stand for crystallization at 25°C After 12 hours, filter with suction and wash with ethanol to obtain a white solid, which is dried. Yield 92.04%, purity 99.23%.
[0032] Purification of isopropanol: volume of isopropanol: 1-(4-methoxyphenyl...
Embodiment 2
[0034] Compound (2) (4.9g) and 100mL of isopropanol were sequentially added into a stainless steel autoclave, sealed, started to pass ammonia gas, heated to 60°C, kept the pressure at 0.4MPa, reacted for 12 hours, and stopped the reaction. After the reactor was cooled to room temperature, it was opened, filtered, and the filter cake was washed with ethanol. After drying, 4.23 g of white solid mp236-239°C was obtained. The yield was 92.06%, and the HPLC purity was 97.15%.
[0035] Ethanol refining: volume of ethanol: 1-(4-methoxyphenyl)-7-oxo-6-[4-(2-oxo-1-piperidinyl)phenyl]-4,5,6, 7-Tetrahydro-1H-pyrazolo[3,4-C]pyridine-3-carboxamide Mass=15ML: 1G, stir and heat to reflux, the system is clear, filter the insoluble matter while it is hot, and stand for crystallization at 25°C After 12 hours, filter with suction and wash with ethanol to obtain a white solid, which is dried. Yield 93.24%, purity 98.92%.
[0036] Purification of isopropanol: volume of isopropanol: 1-(4-methoxyp...
Embodiment 3
[0038] Compound (2) (4.9g) and 200mL of methanol were sequentially added into a stainless steel autoclave, sealed, started to pass ammonia gas, heated to 80°C, kept the pressure at 0.2MPa, reacted for 4 hours, and stopped the reaction. After the reactor was cooled to room temperature, it was opened, filtered, and the filter cake was washed with ethanol. After drying, 4.27 g of white solid mp236-239°C was obtained. The yield was 92.93%, and the HPLC purity was 97.51%.
[0039] Ethanol refining: volume of ethanol: 1-(4-methoxyphenyl)-7-oxo-6-[4-(2-oxo-1-piperidinyl)phenyl]-4,5,6, 7-Tetrahydro-1H-pyrazolo[3,4-C]pyridine-3-carboxamide Mass=60ML: 1G, stir and heat to reflux, the system is clear, filter the insoluble matter while it is hot, and stand for crystallization at 25°C After 12 hours, filter with suction and wash with ethanol to obtain a white solid, which is dried. Yield 90.23%, purity 99.54%.
[0040] Purification of isopropanol: volume of isopropanol: 1-(4-methoxypheny...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com