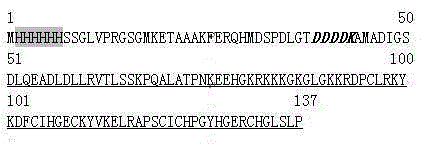Polypeptides with anticoagulation activity screened by phage display technique
An anticoagulant activity and anticoagulant technology, applied in the fields of peptides, blood diseases, peptide/protein components, etc., can solve the problems of low efficiency and large workload, achieve easy transportation, improve the success rate, and broad clinical application value and foreground effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1. pET-30a / (His) 6 Construction of / HB-EGF protein expression vector
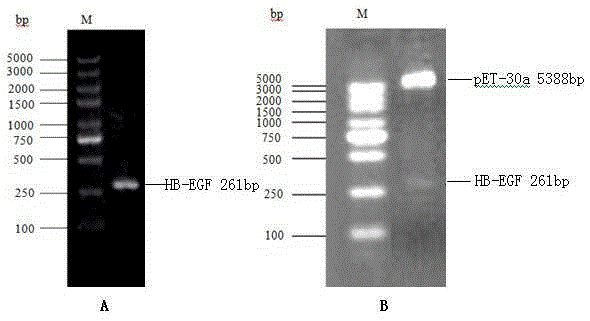
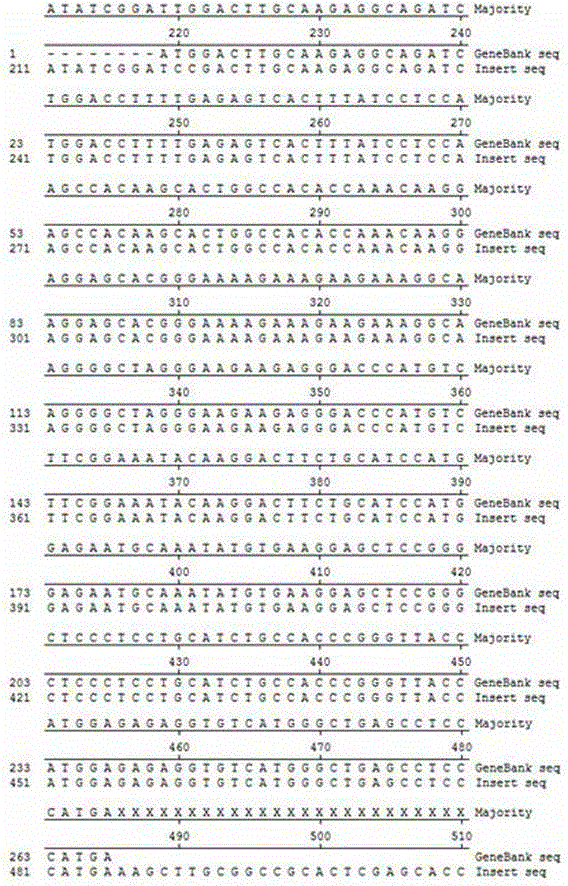
[0025] Extract the fragment encoding HB-EGF (aa63–149, GenBank registration number NM_001945) from GeneBank, and design PCR primers: Forwardprimer5’-CG GGATCC GACTTGCAAGAGGCAGAT-3' (SEQ.ID.NO.3) and Reverseprimer5'-CC AAGCTT TCATGGGAGGCTCAGCCC-3' (SEQ.ID.NO.4), the underlined parts are restriction enzyme cutting sites BamHI and HindIII respectively. After the PCR product and vector pET-30a (Novagen, #69909-3) were digested with BamHI (NEWENGLANDBioLabs, #R0136S) and HindIII (NEWENGLANDBioLabs, #R0104S) at 37°C for 3 h, T4 DNA ligase (NEWENGLANDBioLabs, #M0202S) was used at 16 ℃ connection for 12h. The ligated product was transformed into DH5α competent cells (full type gold, CD201), and then the transformed product was spread on a kanamycin-resistant (50 μg / ml) LB plate and cultured until a single colony grew out, and a single colony was picked, Extract the plasmid for enzyme digestion ...
Embodiment 2
[0027] Example 2. (His) 6 -Induced expression, purification, digestion and verification of HB-EGF
[0028] (His) 6 -Induced expression of HB-EGF: the recombinant plasmid pET-30a / (His) 6 / HB-EGF transforms the host strain BL21 (DE3) (full gold, CD601), utilizes the kanamycin-resistant LB plate to screen the recombinant, picks a single colony and cultures it in the LB liquid medium containing kanamycin until OD 600 = 0.5 to 0.8. The culture was inoculated in LB liquid medium at a volume ratio of 1:50, and cultured to OD at 37°C with vigorous shaking 600 =0.5-0.8, add IPTG (Amresco, #0478) at a final concentration of 0.8mM and induce at 25°C for 12h.
[0029] Nickel column affinity chromatography purification (His) 6 -HB-EGF: Centrifuge the bacterial solution at 6,000rpm for 5min, remove the supernatant, and resuspend the bacterial pellet in the lysate (50mM Tris–HCl, 20mM imidazole, 100mM NaCl, 10 % glycerol, 1% Triton, 1 mM protease inhibitor PMSF, 1 mg / ml lysozyme, pH 8...
Embodiment 3
[0034] Example 3. Phage display panning for biologically active peptides specifically binding to HB-EGF
[0035] The phage panning process is as follows: Figure 5 shown.
[0036] (1) Target molecule immobilization: 600 μl of target molecule solution (dissolved in 0.1M NaHCO 3 PH8.6) was added to a six-well plate, placed on a shaker and shaken slightly, and incubated overnight at 4°C. The target molecule solution was removed and washed 6 times with TBST (50 mM Tris-HCl pH 7.5, 150 mM NaCl, 0.1% [v / v] Tween-20). Finally, the blocking solution (0.1M NaHCO 3 PH8.6, 5mg / mlBSA, 0.02%NaN 3 ) closed for 1h.
[0037] (2) Binding of the phage random peptide library to the target molecule: remove the blocking solution, and wash with TBST (0.1% [v / v] Tween-20) for 10 times. After the phage library or the amplified phage is diluted with TBST (0.1% [v / v] Tween-20), the theoretical value of the copy number of the phage is 10 9 ~10 11 In between, the diluted phage was added to the si...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com