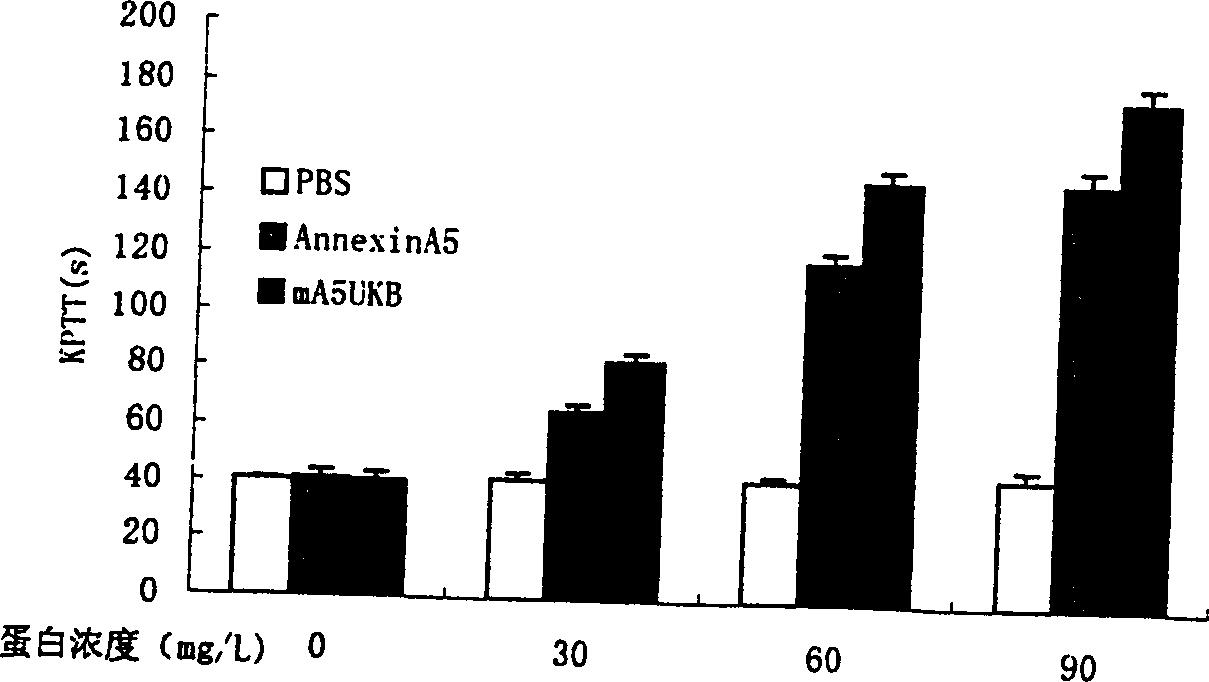
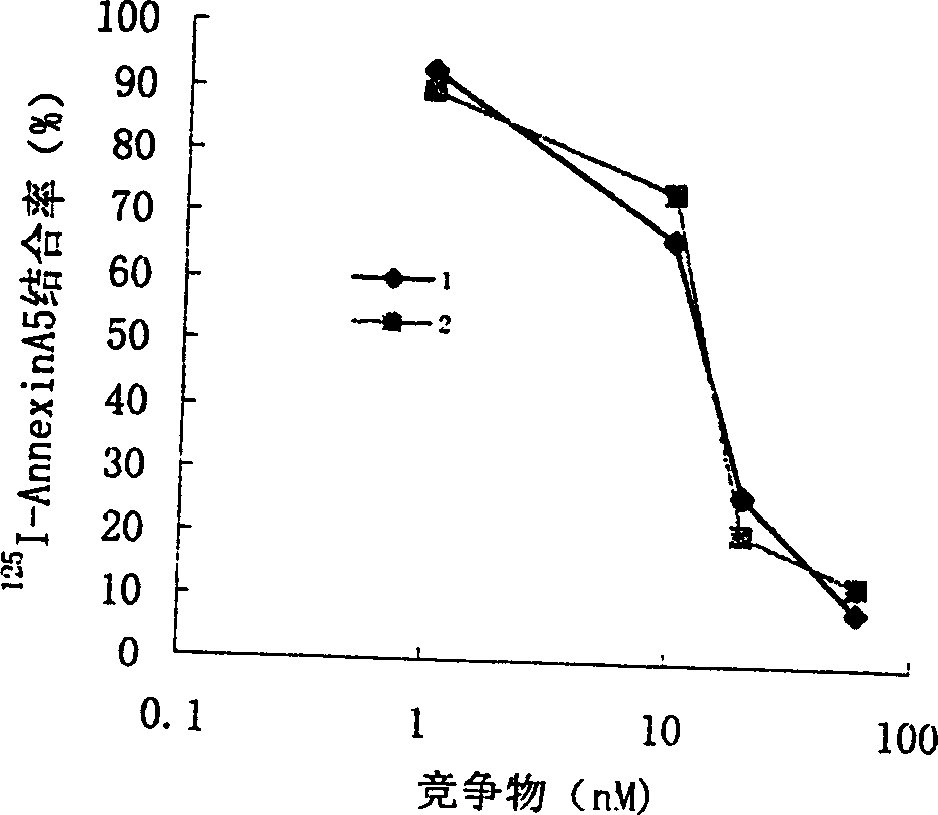
Anticoagulation and thrombolytic thrombus target fusion mA5UKB
A dual-function, fusion protein technology, applied in the field of pharmaceutical bioengineering, can solve the problems of treatment failure, large amount of treatment, short half-life, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] Embodiment 1 constructs mAnxA5 mutant gene
[0073] The gene sequence of human AnnexinA5 was obtained from human placenta tissue by RT-PCR method. In order to facilitate further operations such as amplification and enzyme digestion, this gene was inserted into plasmid pUC119 to obtain recombinant plasmid pUC119-AnxA5. (Referring to relevant chapters of "Molecular Cloning", Science Press, published in 1992).
[0074] 1. Mutate the "V-G-D" sequence at position 142-144 in the AnnexinA5 gene to the "R-G-D" sequence
[0075] In a 100μl PCR reaction system, add 2μl (15pmol / μl) of primers P1 and P2, 1μl (50ng / μl) of pUC119-AnxA5 template DNA, 15mM MgCl 2 10 μl, 3 units of high-fidelity pfu DNA polymerase, 5 μl of 10mM dNTP, 10 μl of 10×PCR buffer, supplemented with double distilled water to 100 μl. The PCR conditions are: the first cycle of denaturation at 94°C for 4 minutes, and the second cycle: denaturation at 94°C for 45 seconds; annealing at 55°C for 45 seconds; extens...
Embodiment 2
[0080] Example 2 Construction of Fusion Gene mA5UKB
[0081] 1. PCR amplification of urokinase B chain gene
[0082] The synthetic urokinase B chain gene was inserted into plasmid pUC119 to obtain recombinant plasmid pUC119-UKB. In 100μl PCR reaction system, add 15pmol / μl of P6, 2μl of P7 primers, 1μl (50ng) of template plasmid DNA pUC119-UKB, 5μl of 10mM dNTP, 10μl of 10×PCR buffer, 15mM MgCl 2 10 μl. The PCR conditions are: the first cycle of denaturation at 94°C for 4 minutes, and the second cycle: denaturation at 94°C for 45 seconds; annealing at 55°C for 45 seconds; extension at 72°C for 1 minute. A total of 30 cycles were performed, and the last cycle was extended at 72°C for 10 minutes. After the reaction, the product was subjected to 1% agarose gel electrophoresis, and the PCR product was recovered from the gel. The obtained PCR product was confirmed to be the urokinase B chain gene (UKB gene) by sequencing.
[0083] 2. Construction of fusion gene mA5UKB gene
[0...
Embodiment 3
[0085] Example 3 Construction of prokaryotic expression plasmid pET28a-mA5UKB and engineering bacteria BL21 (mA5UKB)
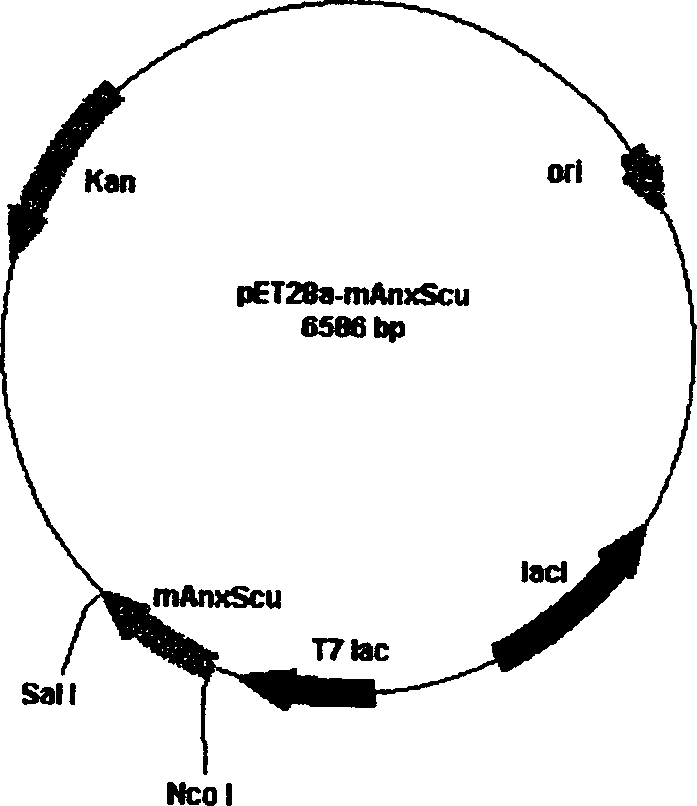
[0086] The invention adopts IPTG-inducible Escherichia coli expression vector pET-28a. The full length of the plasmid is 5396bp (base pairs), containing phage T7 promoter, Luc repressor gene LucI, plasmid replication origin (ori) and Kanna resistance gene (kan r ). The fusion gene mA5UKB obtained in Example 2 was double digested with Nco I and Sal I, and the digested fragments were recovered from the gel. Mix it with the vector pET28a (molecule number: 5:1) that was also digested with Nco I and Sal I, add T4 ligase to the 20ul ligation system, and connect overnight at 14°C to obtain the recombinant plasmid pET28a-mA5UKB ( figure 1 ).
[0087] Preparation of competent cells BL21(DE3): Pick a single colony of Escherichia coli BL21(DE3) and put it in 3ml LB medium (containing 1% tryptone, 0.5% yeast extract and 1% sodium chloride, pH7.0) Incubate overnight a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com