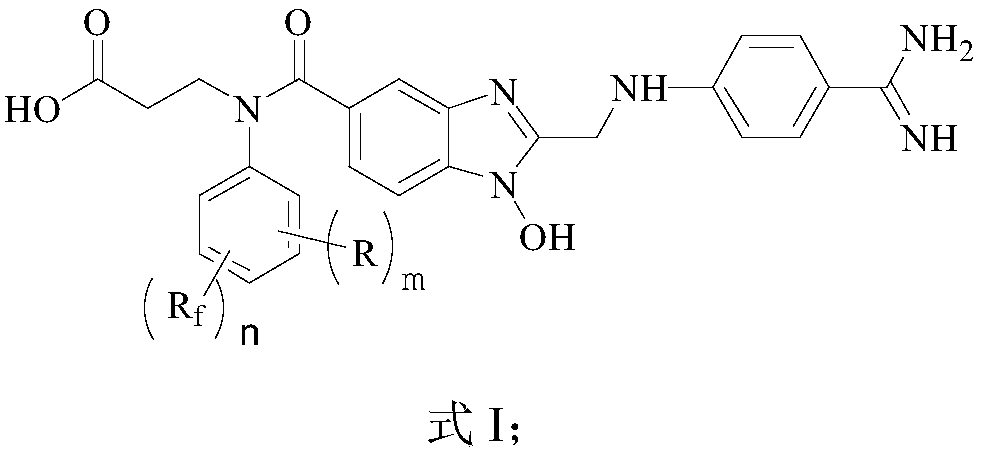
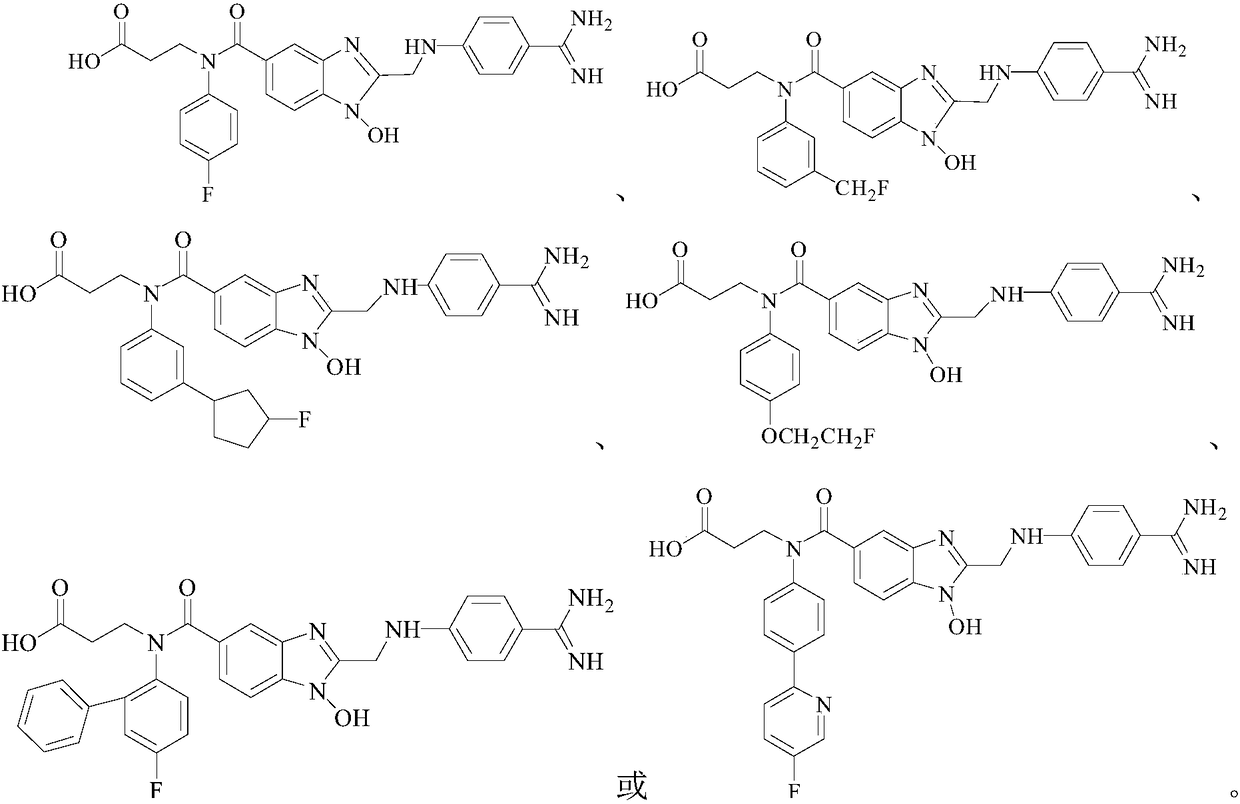
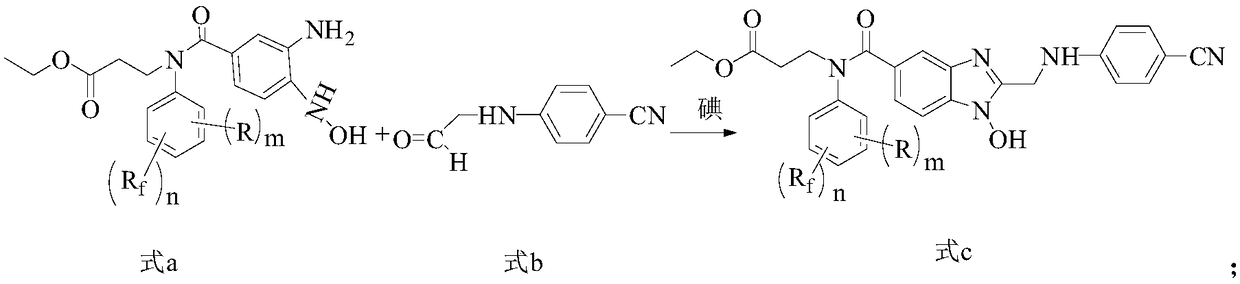
Fluorine-containing benzimidazole compounds and preparation method and application thereof
A technology of benzimidazole and compound, applied in the field of compound synthesis, can solve the problems of long time required, long preparation process, low yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0089] In this example, the target compound A was prepared by the following preparation method: Its preparation method is as follows:
[0090] (1) Add ethyl acrylate dropwise to the N,N-dimethylformamide organic solvent of p-fluoroaniline at room temperature, the molar ratio of p-fluoroaniline to ethyl acrylate is 1:1.1, and add An appropriate amount of diethylamine was reacted at room temperature for 3 hours to obtain compound 1, and the reaction formula was as follows:
[0091]
[0092] (2) 3-nitro-4-chloro-benzoic acid and hydroxylamine aqueous solution were reacted for 2 hours at a temperature of 40° C., wherein the molar ratio of 3-nitro-4-chloro-benzoic acid to hydroxylamine was 1:1.2 to obtain 3-nitro-4-hydroxylamino-benzoic acid compound, the reaction formula is as follows:
[0093]
[0094] (3) 3-nitro-4-hydroxyamino-benzoic acid compound and thionyl chloride were reacted in dichloromethane solvent at 70°C for 8 hours at a molar ratio of 1:1.1 under the actio...
Embodiment 2
[0112] In this example, the target compound B was prepared by the following preparation method: Its preparation method is as follows:
[0113] (1) Add ethyl acrylate dropwise to the N,N-dimethylformamide organic solvent of 3-fluoromethylaniline at room temperature, the molar ratio of 3-fluoromethylaniline to ethyl acrylate is 1 : 1.2, and add an appropriate amount of diethylamine to the reaction, react at room temperature for 5 hours, obtain compound 3, and the reaction formula is as follows:
[0114]
[0115] (2) 3-nitro-4-chloro-benzoic acid and hydroxylamine aqueous solution were reacted for 1 hour at a temperature of 50° C., wherein the molar ratio of 3-nitro-4-chloro-benzoic acid to hydroxylamine was 1:1.3 to obtain 3-nitro-4-hydroxylamino-benzoic acid compound, the reaction formula is as follows:
[0116]
[0117] (3) 3-nitro-4-hydroxyamino-benzoic acid compound and thionyl chloride react at 60°C in dichloromethane solvent under the action of catalyst benzyltrie...
Embodiment 3
[0135] In this example, the target compound C was prepared by the following preparation method: Its preparation method is as follows:
[0136] (1) Add ethyl acrylate dropwise to the N,N-dimethylformamide organic solvent of the aniline compound shown in formula II at room temperature, the molar ratio of the aniline compound shown in formula II to ethyl acrylate is 1:1.3 , and add an appropriate amount of diethylamine to the reaction, and react at room temperature for 8 hours to obtain compound 4, the reaction formula is as follows:
[0137]
[0138] (2) 3-nitro-4-chloro-benzoic acid and hydroxylamine aqueous solution were reacted for 2 hours at a temperature of 40° C., wherein the molar ratio of 3-nitro-4-chloro-benzoic acid to hydroxylamine was 1:1.2 to obtain 3-nitro-4-hydroxylamino-benzoic acid compound, the reaction formula is as follows:
[0139]
[0140] (3) 3-nitro-4-hydroxyamino-benzoic acid compound and thionyl chloride were reacted in dichloromethane solvent ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com